Use of cassette dosing in sandwich-cultured rat and human hepatocytes to identify drugs that inhibit bile acid transport
- PMID: 19706322
- PMCID: PMC3129609
- DOI: 10.1016/j.tiv.2009.08.009
Use of cassette dosing in sandwich-cultured rat and human hepatocytes to identify drugs that inhibit bile acid transport
Abstract
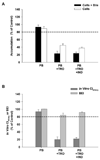
Hepatocellular accumulation of bile acids due to inhibition of the canalicular bile salt export pump (BSEP/ABCB11) is one proposed mechanism of drug-induced liver injury (DILI). Some hepatotoxic compounds also are potent inhibitors of bile acid uptake by Na(+)-dependent taurocholate cotransporting polypeptide (NTCP/SLC10A1). This study used a cassette dosing approach in rat and human sandwich-cultured hepatocytes (SCH) to determine whether known or suspected hepatotoxic drugs inhibit bile acid transport individually or in combination. [(3)H]-Taurocholate served as the NTCP/BSEP probe substrate. Individually, cyclosporin A and rifampin decreased taurocholate in vitro biliary clearance (Cl(biliary)) and biliary excretion index (BEI) by more than 20% in rat SCH, suggesting that these drugs primarily inhibited canalicular efflux. In contrast, ampicillin, carbenicillin, cloxacillin, nafcillin, oxacillin, carbamazepine, pioglitazone, and troglitazone decreased the in vitro Cl(biliary) by more than 20% with no notable change in BEI, suggesting that these drugs primarily inhibited taurocholate uptake. Cassette dosing (n=2-4 compounds per cassette) in rat SCH yielded similar findings, and results in human SCH were consistent with rat SCH. In summary, cassette dosing in SCH is a useful in vitro approach to identify compounds that inhibit the hepatic uptake and/or excretion of bile acids, which may cause DILI.
Conflict of interest statement
K.L.R.B. is a co-founder and Chair of the Scientific Advisory Board of Qualyst, Inc. Qualyst, Inc. has exclusively licensed the sandwich-cultured hepatocyte technology for quantification of biliary excretion (B-CLEAR®).
Figures













References
-
- Bakke OM, Manocchia M, de Abajo F, Kaitin KI, Lasagna L. Drug safety discontinuations in the United Kingdom, the United States, and Spain from 1974 through 1993: a regulatory perspective. Clinical Pharmacology and Therapeutics. 1995;58:108–117. - PubMed
-
- Bénichou C. Criteria of drug-induced liver disorders. Report of an international consensus meeting. Journal of Hepatology. 1990;11:272–276. - PubMed
-
- Crocenzi FA, Sisti A, Pellegrino JM, Roma MG. Role of bile salts in colchicine-induced hepatotoxicity. Implications for hepatocellular integrity and function. Toxicology. 1997;121:127–142. - PubMed
-
- Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharmaceutical Research. 1993;10:1093–1095. - PubMed
-
- EMEA. Non-clinical guideline on drug-induced hepatotoxicity. 2008 Available at: http://www.emea.europa.eu/pdfs/human/swp/15011506en.pdf.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

