Reduction of seizures by transplantation of cortical GABAergic interneuron precursors into Kv1.1 mutant mice
- PMID: 19706400
- PMCID: PMC2741275
- DOI: 10.1073/pnas.0900141106
Reduction of seizures by transplantation of cortical GABAergic interneuron precursors into Kv1.1 mutant mice
Abstract
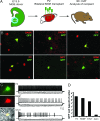
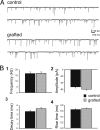
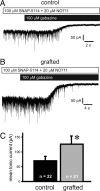
Epilepsy, a disease characterized by abnormal brain activity, is a disabling and potentially life-threatening condition for nearly 1% of the world population. Unfortunately, modulation of brain excitability using available antiepileptic drugs can have serious side effects, especially in the developing brain, and some patients can only be improved by surgical removal of brain regions containing the seizure focus. Here, we show that bilateral transplantation of precursor cells from the embryonic medial ganglionic eminence (MGE) into early postnatal neocortex generates mature GABAergic interneurons in the host brain. In mice receiving MGE cell grafts, GABA-mediated synaptic and extrasynaptic inhibition onto host brain pyramidal neurons is significantly increased. Bilateral MGE cell grafts in epileptic mice lacking a Shaker-like potassium channel (a gene mutated in one form of human epilepsy) resulted in significant reductions in the duration and frequency of spontaneous electrographic seizures. Our findings suggest that MGE-derived interneurons could be used to ameliorate abnormal excitability and possibly act as an effective strategy in the treatment of epilepsy.
Conflict of interest statement
Conflict of interest statement: S.C.B., J.L.R.R, and A.A.B are cofounders of, and have a financial interest in, Neurona Therapeutics.
Figures



References
-
- Li T, et al. Suppression of kindling epileptogenesis by adenosine releasing stem cell-derived brain implants. Brain. 2007;130:1276–1288. - PubMed
-
- de Lanerolle NC, Kim JH, Robbins RJ, Spencer DD. Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res. 1989;495:387–395. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

