Metabolic labeling and direct imaging of choline phospholipids in vivo
- PMID: 19706413
- PMCID: PMC2741251
- DOI: 10.1073/pnas.0907864106
Metabolic labeling and direct imaging of choline phospholipids in vivo
Abstract
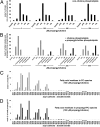
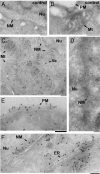
Choline (Cho)-containing phospholipids are the most abundant phospholipids in cellular membranes and play fundamental structural as well as regulatory roles in cell metabolism and signaling. Although much is known about the biochemistry and metabolism of Cho phospholipids, their cell biology has remained obscure, due to the lack of methods for their direct microscopic visualization in cells. We developed a simple and robust method to label Cho phospholipids in vivo, based on the metabolic incorporation of the Cho analog propargylcholine (propargyl-Cho) into phospholipids. The resulting propargyl-labeled phospholipid molecules can be visualized with high sensitivity and spatial resolution in cells via a Cu(I)-catalyzed cycloaddition reaction between the terminal alkyne group of propargyl-Cho and a labeled azide. Total lipid analysis of labeled cells shows strong incorporation of propargyl-Cho into all classes of Cho phospholipids; furthermore, the fatty acid composition of propargyl-Cho-labeled phospholipids is very similar to that of normal Cho phospholipids. We demonstrate the use of propargyl-Cho in cultured cells, by imaging phospholipid synthesis, turnover, and subcellular localization by both fluorescence and electron microscopy. Finally, we use propargyl-Cho to assay microscopically phospholipid synthesis in vivo in mouse tissues.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Vance JE, Vance DE. Phospholipid biosynthesis in mammalian cells. Biochem Cell Biol. 2004;82:113–128. - PubMed
-
- Nagan N, Zoeller RA. Plasmalogens: Biosynthesis and functions. Prog Lipid Res. 2001;40:199–229. - PubMed
-
- Weiss SB, Smith SW, Kennedy EP. The enzymatic formation of lecithin from cytidine diphosphate choline and D-1,2-diglyceride. J Biol Chem. 1958;231:53–64. - PubMed
-
- Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem Int Ed Engl. 2002;41:2596–2599. - PubMed
-
- Tornoe CW, Christensen C, Meldal M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper (i) -catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J Org Chem. 2002;67:3057–3064. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

