High pressure chemistry in the H2-SiH4 system
- PMID: 19706419
- PMCID: PMC2736422
- DOI: 10.1073/pnas.0907729106
High pressure chemistry in the H2-SiH4 system
Abstract
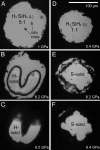
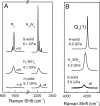
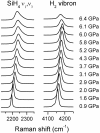
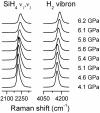
Understanding the behavior of hydrogen-rich systems at extreme conditions has significance to both condensed matter physics, where it may provide insight into the metallization and superconductivity of element one, and also to applied research areas, where it can provide guidance for designing improved hydrogen storage materials for transportation applications. Here we report the high-pressure study of the SiH4-H2 binary system up to 6.5 GPa at 300 K in a diamond anvil cell. Raman measurements indicate significant intermolecular interactions between H2 and SiH4. We found that the H2 vibron frequency is softened by the presence of SiH4 by as much as 40 cm(-1) for the fluid with 50 mol% H2 compared with pure H2 fluid at the same pressures. In contrast, the Si-H stretching modes of SiH4 shift to higher frequency in the mixed fluid compared with pure SiH4. Pressure-induced solidification of the H2-SiH4 fluid shows a binary eutectic point at 72(+/-2) mol% H2 and 6.1(+/-0.1) GPa, above which the fluid crystallizes into a mixture of two nearly end-member solids. Neither solid has a pure end-member composition, with the silane-rich solid containing 0.5-1.5 mol% H2 and the hydrogen-rich solid containing 0.5-1 mol% SiH4. These two crystalline phases can be regarded as doped hydrogen-dominant compounds. We were able to superpressurize the sample by 0.2-0.4 GPa above the eutectic before complete crystallization, indicating extended metastability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Ashcroft NW. Hydrogen dominant metallic alloys: High temperature superconductors? Phys Rev Lett. 2004;92:187002. - PubMed
-
- Eremets MI, Trojan IA, Medvedev SA, Tse JS, Yao Y. Superconductivity in hydrogen dominant materials: Silane. Science. 2008;319:1506–1509. - PubMed
-
- Mao WL, Koh CA, Sloan ED. Clathrate hydrates under pressure. Physics Today. 2007:42–47.
LinkOut - more resources
Full Text Sources

