Human deoxyhypusine hydroxylase, an enzyme involved in regulating cell growth, activates O2 with a nonheme diiron center
- PMID: 19706422
- PMCID: PMC2736468
- DOI: 10.1073/pnas.0904553106
Human deoxyhypusine hydroxylase, an enzyme involved in regulating cell growth, activates O2 with a nonheme diiron center
Abstract
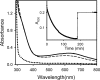
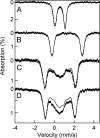
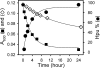
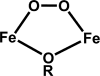
Deoxyhypusine hydroxylase is the key enzyme in the biosynthesis of hypusine containing eukaryotic translation initiation factor 5A (eIF5A), which plays an essential role in the regulation of cell proliferation. Recombinant human deoxyhypusine hydroxylase (hDOHH) has been reported to have oxygen- and iron-dependent activity, an estimated iron/holoprotein stoichiometry of 2, and a visible band at 630 nm responsible for the blue color of the as-isolated protein. EPR, Mössbauer, and XAS spectroscopic results presented herein provide direct spectroscopic evidence that hDOHH has an antiferromagnetically coupled diiron center with histidines and carboxylates as likely ligands, as suggested by mutagenesis experiments. Resonance Raman experiments show that its blue chromophore arises from a (mu-1,2-peroxo)diiron(III) center that forms in the reaction of the reduced enzyme with O2, so the peroxo form of hDOHH is unusually stable. Nevertheless we demonstrate that it can carry out the hydroxylation of the deoxyhypusine residue present in the elF5A substrate. Despite a lack of sequence similarity, hDOHH has a nonheme diiron active site that resembles both in structure and function those found in methane and toluene monooxygenases, bacterial and mammalian ribonucleotide reductases, and stearoyl acyl carrier protein Delta9-desaturase from plants, suggesting that the oxygen-activating diiron motif is a solution arrived at by convergent evolution. Notably, hDOHH is the only example thus far of a human hydroxylase with such a diiron active site.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Park MH, Wolff EC, Leea YB, Folk JE. Antiproliferative effects of inhibitors of deoxyhypusine synthase: Inhibition of growth of Chinese hamster ovary cells by guanul diamines. J Biol Chem. 1994;269:27827–27832. - PubMed
-
- Hanauske-Abel HM, et al. Inhibition of the G1-S transition of the cell cycle by inhibitors of deoxyhypusine hydroxylation. Biochim Biophys Acta. 1994;1221:115–124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

