The inverse and direct Hofmeister series for lysozyme
- PMID: 19706429
- PMCID: PMC2741236
- DOI: 10.1073/pnas.0907616106
The inverse and direct Hofmeister series for lysozyme
Abstract
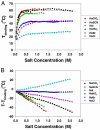
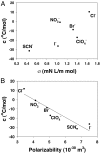
Anion effects on the cloud-point temperature for the liquid-liquid phase transition of lysozyme were investigated by temperature gradient microfluidics under a dark field microscope. It was found that protein aggregation in salt solutions followed 2 distinct Hofmeister series depending on salt concentration. Namely, under low salt conditions the association of anions with the positively charged lysozyme surface dominated the process and the phase transition temperature followed an inverse Hofmeister series. This inverse series could be directly correlated to the size and hydration thermodynamics of the anions. At higher salt concentrations, the liquid-liquid phase transition displayed a direct Hofmeister series that correlated with the polarizability of the anions. A simple model was derived to take both charge screening and surface tension effects into account at the protein/water interface. Fitting the thermodynamic data to this model equation demonstrated its validity in both the high and low salt regimes. These results suggest that in general positively charged macromolecular systems should show inverse Hofmeister behavior only at relatively low salt concentrations, but revert to a direct Hofmeister series as the salt concentration is increased.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Benedek GB. Cataract as a protein condensation disease: The proctor lecture. Invest Ophth Vis Sci. 1997;38:1911–1921. - PubMed
-
- Benedek GB, Pande J, Thurston GM, Clark JI. Theoretical and experimental basis for the inhibition of cataract. Prog Retin Eye Res. 1999;18:391–402. - PubMed
-
- Dobson CM. Protein folding and misfolding. Nature. 2003;426:884–890. - PubMed
-
- Gaggelli E, Kozlowski H, Valensin D, Valensin G. Copper homeostasis and neurodegenerative disorders (Alzheimer's, prion, and Parkinson's diseases and amyotrophic lateral sclerosis) Chem Rev. 2006;106:1995–2044. - PubMed
-
- Curtis RA, Prausnitz JM, Blanch HW. Protein-protein and protein-salt interactions in aqueous protein solutions containing concentrated electrolytes. Biotechnol Bioeng. 1998;57:11–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

