Reactions of ozone with human skin lipids: sources of carbonyls, dicarbonyls, and hydroxycarbonyls in indoor air
- PMID: 19706436
- PMCID: PMC2872416
- DOI: 10.1073/pnas.0904498106
Reactions of ozone with human skin lipids: sources of carbonyls, dicarbonyls, and hydroxycarbonyls in indoor air
Abstract
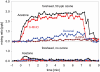
This study has used proton transfer reaction-mass spectrometry (PTR-MS) for direct air analyses of volatile products resulting from the reactions of ozone with human skin lipids. An initial series of small-scale in vitro and in vivo experiments were followed by experiments conducted with human subjects in a simulated office. The latter were conducted using realistic ozone mixing ratios (approximately 15 ppb with occupants present). Detected products included mono- and bifunctional compounds that contain carbonyl, carboxyl, or alpha-hydroxy ketone groups. Among these, three previously unreported dicarbonyls have been identified, and two previously unreported alpha-hydroxy ketones have been tentatively identified. The compounds detected in this study (excepting acetone) have been overlooked in surveys of indoor pollutants, reflecting the limitations of the analytical methods routinely used to monitor indoor air. The results are fully consistent with the Criegee mechanism for ozone reacting with squalene, the single most abundant unsaturated constituent of skin lipids, and several unsaturated fatty acid moieties in their free or esterified forms. Quantitative product analysis confirms that squalene is the major scavenger of ozone at the interface between room air and the human envelope. Reactions between ozone and human skin lipids reduce the mixing ratio of ozone in indoor air, but concomitantly increase the mixing ratios of volatile products and, presumably, skin surface concentrations of less volatile products. Some of the volatile products, especially the dicarbonyls, may be respiratory irritants. Some of the less volatile products may be skin irritants.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Weschler CJ. Ozone in indoor environments: Concentration and chemistry. Indoor Air. 2000;10:269–288. - PubMed
-
- Britigan N, Alshawa A, Nizkorodov SA. Quantification of ozone levels in indoor environments generated by ionization and ozonolysis air purifiers. J Air Waste Manage Assoc. 2006;56:601–610. - PubMed
-
- Jacober C, Phillips T. Evaluation of Ozone Emissions from Portable Indoor Air Cleaners: Electrostatic Precipitators and Ionizers.Staff Technical Report to the California Air Resources Board, February 2008. 2008. Availabe at http://www.arb.ca.gov/research/indoor/esp_report .pdf. Accessed August 2, 2009.
-
- Liu XY, Mason M, Krebs K, Sparks L. Full-scale chamber investigation and simulation of air freshener emissions in the presence of ozone. Environ Sci Technol. 2004;38:2802–2812. - PubMed
-
- Singer BC, et al. Indoor secondary pollutants from cleaning product and air freshener use in the presence of ozone. Atmos Environ. 2006;40:6696–6710. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

