Measuring prions by bioluminescence imaging
- PMID: 19706444
- PMCID: PMC2736416
- DOI: 10.1073/pnas.0907339106
Measuring prions by bioluminescence imaging
Abstract
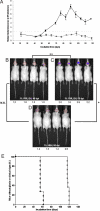
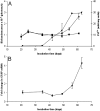
Prions are infectious proteins that cause fatal neurodegenerative diseases. Because astrocytic gliosis marked by the deposition of fibrils composed of GFAP is a prominent feature of prion disease, we asked whether GFAP might be used as a surrogate marker for prions. To interrogate this posit, we inoculated prions into transgenic (Tg) mice expressing luciferase (luc) under the GFAP gene (Gfap) promoter, denoted Tg(Gfap-luc) mice. Weekly noninvasive, bioluminescence imaging (BLI) detected an increase in light emitted from the brains of Tg(Gfap-luc) mice at approximately 55 d after inoculation and approximately 62 d before neurologic deficits appeared. To determine whether BLI could be used as a proxy bioassay for prion infectivity, we performed endpoint titrations of prions in Tg(Gfap-luc) mice. BLI bioassays were as or more sensitive than those determined by the onset of neurological dysfunction, and were completed in approximately half the time. Our studies argue that BLI is likely to be a suitable surrogate for measuring prion infectivity, and might be useful in the study of Tg mouse models for other neurodegenerative illnesses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Chesebro B. Introduction to the transmissible spongiform encephalopathies or prion diseases. Br Med Bull. 2003;66:1–20. - PubMed
-
- Weissmann C. The state of the prion. Nat Rev Microbiol. 2004;2:861–871. - PubMed
-
- Caughey B, Baron GS. Prions and their partners in crime. Nature. 2006;443:803–810. - PubMed
-
- Prusiner SB. Prions. In: Knipe DM, et al., editors. Fields Virology. 5th Ed. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 3059–3092.
-
- Aguzzi A, Sigurdson C, Heikenwaelder M. Molecular mechanisms of prion pathogenesis. Annu Rev Pathol. 2008;3:11–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

