Melatonin modulates visual function and cell viability in the mouse retina via the MT1 melatonin receptor
- PMID: 19706469
- PMCID: PMC2736407
- DOI: 10.1073/pnas.0904400106
Melatonin modulates visual function and cell viability in the mouse retina via the MT1 melatonin receptor
Abstract
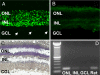
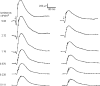
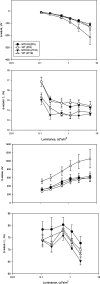
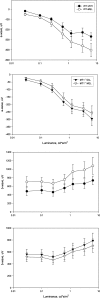
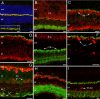
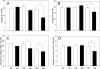
A clear demonstration of the role of melatonin and its receptors in specific retinal functions is lacking. The present study investigated the distribution of MT1 receptors within the retina, and the scotopic and photopic electroretinograms (ERG) and retinal morphology in wild-type (WT) and MT1 receptor-deficient mice. MT1 receptor transcripts were localized in photoreceptor cells and in some inner retinal neurons. A diurnal rhythm in the dark-adapted ERG responses was observed in WT mice, with higher a- and b-wave amplitudes at night, but this rhythm was absent in mice lacking MT1 receptors. Injection of melatonin during the day decreased the scotopic response threshold and the amplitude of the a- and b-waves in the WT mice, but not in the MT1(-/-) mice. The effects of MT1 receptor deficiency on retinal morphology was investigated at three different ages (3, 12, and 18 months). No differences between MT1(-/-) and WT mice were observed at 3 months of age, whereas at 12 months MT1(-/-) mice have a significant reduction in the number of photoreceptor nuclei in the outer nuclear layer compared with WT controls. No differences were observed in the number of cells in inner nuclear layer or in ganglion cells at 12 months of age. At 18 months, the loss of photoreceptor nuclei in the outer nuclear layer was further accentuated and the number of ganglion cells was also significantly lower than that of controls. These data demonstrate the functional significance of melatonin and MT1 receptors in the mammalian retina and create the basis for future studies on the therapeutic use of melatonin in retinal degeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Dubocovich ML, Markowska M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine. 2005;27:101–110. - PubMed
-
- Cahill GM, Besharse JC. Circadian clock functions localized in Xenopus retinal photoreceptors. Neuron. 1993;10:573–577. - PubMed
-
- Liu C, Fukuhara C, Wessel JH, Iuvone PM, Tosini G. Localization of Aa-nat mRNA in the rat retina by fluorescence in situ hybridization and laser capture microdissection. Cell Tissue Res. 2004;315:197–201. - PubMed
-
- Ivanova TN, Iuvone PM. Circadian rhythm and photic control of cAMP level in chick retinal cell cultures: A mechanism for coupling the circadian oscillator to the melatonin-synthesizing enzyme, arylalkylamine N-acetyltransferase, in photoreceptor cells. Brain Res. 2003;991:96–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

