Two-photon excitation of channelrhodopsin-2 at saturation
- PMID: 19706471
- PMCID: PMC2736443
- DOI: 10.1073/pnas.0907084106
Two-photon excitation of channelrhodopsin-2 at saturation
Abstract
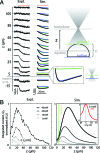
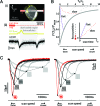
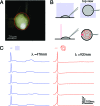
We demonstrate that channelrhodopsin-2 (CR), a light-gated ion channel that is conventionally activated by using visible-light excitation, can also be activated by using IR two-photon excitation (TPE). An empirical estimate of CR's two-photon absorption cross-section at lambda = 920 nm is presented, with a value (260 +/- 20 GM) indicating that TPE stimulation of CR photocurrents is not typically limited by intrinsic molecular excitability [1 GM = 10(-50)(cm4 s)/photon]. By using direct physiological measurements of CR photocurrents and a model of ground-state depletion, we evaluate how saturation of CR's current-conducting state influences the spatial resolution of focused TPE photostimulation, and how photocurrents stimulated by using low-power scanning TPE temporally summate. We show that TPE, like visible-light excitation, can be used to stimulate action potentials in cultured CR-expressing neurons.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. - PubMed
-
- Berndt A, Yizhar O, Gunaydin LA, Hegemann P, Deisseroth K. Bi-stable neural state switches. Nat Neurosci. 2009;12:229–234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

