Heme oxygenase-1 affords protection against noncerebral forms of severe malaria
- PMID: 19706490
- PMCID: PMC2728109
- DOI: 10.1073/pnas.0903419106
Heme oxygenase-1 affords protection against noncerebral forms of severe malaria
Abstract
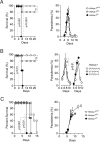
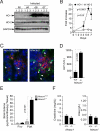
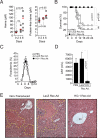
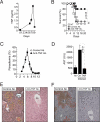
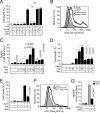
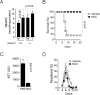
Infection by Plasmodium, the causative agent of malaria, is associated with hemolysis and therefore with release of hemoglobin from RBC. Under inflammatory conditions, cell-free hemoglobin can be oxidized, releasing its heme prosthetic groups and producing deleterious free heme. Here we demonstrate that survival of a Plasmodium-infected host relies strictly on its ability to prevent the cytotoxic effects of free heme via the expression of the heme-catabolyzing enzyme heme oxygenase-1 (HO-1; encoded by the Hmox1 gene). When infected with Plasmodium chabaudi chabaudi (Pcc), wild-type (Hmox1(+/+)) BALB/c mice resolved infection and restored homeostasis thereafter (0% lethality). In contrast, HO-1 deficient (Hmox1(-/-)) BALB/c mice developed a lethal form of hepatic failure (100% lethality), similar to the one occurring in Pcc-infected DBA/2 mice (75% lethality). Expression of HO-1 suppresses the pro-oxidant effects of free heme, preventing it from sensitizing hepatocytes to undergo TNF-mediated programmed cell death by apoptosis. This cytoprotective effect, which inhibits the development of hepatic failure in Pcc-infected mice without interfering with pathogen burden, is mimicked by pharmacological antioxidants such as N-acetylcysteine (NAC). When administered therapeutically, i.e., after Pcc infection, NAC suppressed the development of hepatic failure in Pcc-infected DBA/2 mice (0% lethality), without interfering with pathogen burden. In conclusion, we describe a mechanism of host defense against Plasmodium infection, based on tissue cytoprotection against free heme and limiting disease severity irrespectively of parasite burden.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Comment in
-
Damage control in host-pathogen interactions.Proc Natl Acad Sci U S A. 2009 Sep 15;106(37):15525-6. doi: 10.1073/pnas.0908451106. Epub 2009 Sep 9. Proc Natl Acad Sci U S A. 2009. PMID: 19805209 Free PMC article. No abstract available.
References
-
- Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature. 2002;415:673–679. - PubMed
-
- Raberg L, Sim D, Read AF. Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science. 2007;318:812–814. - PubMed
-
- Ferreira A, Balla J, Jeney V, Balla G, Soares MP. A central role for free heme in the pathogenesis of severe malaria: The missing link? J Mol Med. 2008;86:1097–1111. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

