Antiestrogen-resistant subclones of MCF-7 human breast cancer cells are derived from a common monoclonal drug-resistant progenitor
- PMID: 19706540
- PMCID: PMC2732824
- DOI: 10.1073/pnas.0907560106
Antiestrogen-resistant subclones of MCF-7 human breast cancer cells are derived from a common monoclonal drug-resistant progenitor
Abstract
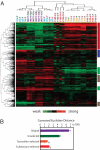
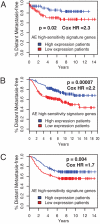
Emergence of antiestrogen-resistant cells in MCF-7 cells during suppression of estrogen signaling is a widely accepted model of acquired breast cancer resistance to endocrine therapy. To obtain insight into the genomic basis of endocrine therapy resistance, we characterized MCF-7 monoclonal sublines that survived 21-day exposure to tamoxifen (T-series sublines) or fulvestrant (F-series sublines) and sublines unselected by drugs (U-series). All T/F-sublines were resistant to the cytocidal effects of both tamoxifen and fulvestrant. However, their responses to the cytostatic effects of fulvestrant varied greatly, and their remarkably diversified morphology showed no correlation with drug resistance. mRNA expression profiles of the U-sublines differed significantly from those of the T/F-sublines, whose transcriptomal responsiveness to fulvestrant was largely lost. A set of genes strongly expressed in the U-sublines successfully predicted metastasis-free survival of breast cancer patients. Most T/F-sublines shared highly homogeneous genomic DNA aberration patterns that were distinct from those of the U-sublines. Genomic DNA of the U-sublines harbored many aberrations that were not found in the T/F-sublines. These results suggest that the T/F-sublines are derived from a common monoclonal progenitor that lost transcriptomal responsiveness to antiestrogens as a consequence of genetic abnormalities many population doublings ago, not from the antiestrogen-sensitive cells in the same culture during the exposure to antiestrogens. Thus, the apparent acquisition of antiestrogen resistance by MCF-7 cells reflects selection of preexisting drug-resistant subpopulations without involving changes in individual cells. Our results suggest the importance of clonal selection in endocrine therapy resistance of breast cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Lacroix M, Leclercq G. Relevance of breast cancer cell lines as models for breast tumours: An update. Breast Cancer Res Treat. 2004;83:249–289. - PubMed
-
- Brodie A, et al. Therapeutic observations in MCF-7 aromatase xenografts. Clin Cancer Res. 2005;11:884s–888s. - PubMed
-
- Hur J, et al. Regulation of expression of BIK proapoptotic protein in human breast cancer cells: p53-dependent induction of BIK mRNA by fulvestrant and proteasomal degradation of BIK protein. Cancer Res. 2006;66:10153–10161. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

