Nitric oxide regulates lung carcinoma cell anoikis through inhibition of ubiquitin-proteasomal degradation of caveolin-1
- PMID: 19706615
- PMCID: PMC2788896
- DOI: 10.1074/jbc.M109.050864
Nitric oxide regulates lung carcinoma cell anoikis through inhibition of ubiquitin-proteasomal degradation of caveolin-1
Abstract
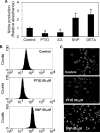
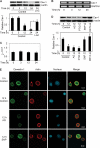
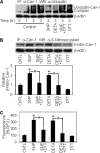
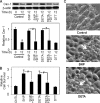
Anoikis, a detachment-induced apoptosis, is a principal mechanism of inhibition of tumor cell metastasis. Tumor cells can acquire anoikis resistance which is frequently observed in metastatic lung cancer. This phenomenon becomes an important obstacle of efficient cancer therapy. Recently, signaling mediators such as caveolin-1 (Cav-1) and nitric oxide (NO) have garnered attention in metastasis research; however, their role and the underlying mechanisms of metastasis regulation are largely unknown. Using human lung carcinoma H460 cells, we show that NO impairs the apoptotic function of the cells after detachment. The NO donors sodium nitroprusside and diethylenetriamine NONOate inhibit detachment-induced apoptosis, whereas the NO inhibitors aminoguanidine and 2-(4-carboxyphenyl) tetramethylimidazoline-1-oxyl-3-oxide promote this effect. Resistance to anoikis in H460 cells is mediated by Cav-1, which is significantly down-regulated after cell detachment through a non-transcriptional mechanism involving ubiquitin-proteasomal degradation. NO inhibits this down-regulation by interfering with Cav-1 ubiquitination through a process that involves protein S-nitrosylation, which prevents its proteasomal degradation and induction of anoikis by cell detachment. These findings indicate a novel pathway for NO regulation of Cav-1, which could be a key mechanism of anoikis resistance in tumor cells.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

