Preclinical evaluation of novel glutamate-urea-lysine analogues that target prostate-specific membrane antigen as molecular imaging pharmaceuticals for prostate cancer
- PMID: 19706750
- PMCID: PMC4114247
- DOI: 10.1158/0008-5472.CAN-09-1682
Preclinical evaluation of novel glutamate-urea-lysine analogues that target prostate-specific membrane antigen as molecular imaging pharmaceuticals for prostate cancer
Abstract
Prostate-specific membrane antigen (PSMA) is expressed in normal human prostate epithelium and is highly up-regulated in prostate cancer. We previously reported a series of novel small molecule inhibitors targeting PSMA. Two compounds, MIP-1072, (S)-2-(3-((S)-1-carboxy-5-(4-iodobenzylamino)pentyl)ureido)pentanedioic acid, and MIP-1095, (S)-2-(3-((S)-1carboxy-5-(3-(4-iodophenyl)ureido)pentyl)ureido)pentanedioic acid, were selected for further evaluation. MIP-1072 and MIP-1095 potently inhibited the glutamate carboxypeptidase activity of PSMA (K(i) = 4.6 +/- 1.6 nmol/L and 0.24 +/- 0.14 nmol/L, respectively) and, when radiolabeled with (123)I, exhibited high affinity for PSMA on human prostate cancer LNCaP cells (K(d) = 3.8 +/- 1.3 nmol/L and 0.81 +/- 0.39 nmol/L, respectively). The association of [(123)I]MIP-1072 and [(123)I]MIP-1095 with PSMA was specific; there was no binding to human prostate cancer PC3 cells, which lack PSMA, and binding was abolished by coincubation with a structurally unrelated NAALADase inhibitor, 2-(phosphonomethyl)pentanedioic acid (PMPA). [(123)I]MIP-1072 and [(123)I]MIP-1095 internalized into LNCaP cells at 37 degrees C. Tissue distribution studies in mice showed 17.3 +/- 6.3% (at 1 hour) and 34.3 +/- 12.7% (at 4 hours) injected dose per gram of LNCaP xenograft tissue, for [(123)I]MIP-1072 and [(123)I]MIP-1095, respectively. [(123)I]MIP-1095 exhibited greater tumor uptake but slower washout from blood and nontarget tissues compared with [(123)I]MIP-1072. Specific binding to PSMA in vivo was shown by competition with PMPA in LNCaP xenografts, and the absence of uptake in PC3 xenografts. The uptake of [(123)I]MIP-1072 and [(123)I]MIP-1095 in tumor-bearing mice was corroborated by single-photon emission computed tomography/computed tomography (SPECT/CT) imaging. PSMA-specific radiopharmaceuticals should provide a novel molecular targeting option for the detection and staging of prostate cancer.
Conflict of interest statement
This work was conducted at Molecular Insight Pharmaceuticals, Inc. S. Hillier, K. Maresca, F. Femia, J. Marquis, C. Zimmerman, J. Barrett, J. Joyal, and J. Babich are employees of Molecular Insight Pharmaceuticals, Inc. W. Eckelman and M. Pomper are consultants for Molecular Insight Pharmaceuticals, Inc.
Figures
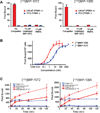
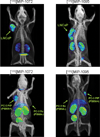
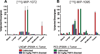
 , PC3 tumor
, PC3 tumor  ) or co-injected with 50 mg/kg PMPA (LNCaP tumor
) or co-injected with 50 mg/kg PMPA (LNCaP tumor  , or PC3 tumor
, or PC3 tumor  ) via the tail vein. Data are expressed as %ID/g.
) via the tail vein. Data are expressed as %ID/g.References
-
- Cancer Facts and Figures. American Cancer Society; 2009.
-
- Hankey BF, Feuer EJ, Clegg LX, et al. Cancer surveillance series: interpreting trends in prostate cancer--part I: Evidence of the effects of screening in recent prostate cancer incidence, mortality, and survival rates. J. Natl. Cancer Inst. 1999;91:1017–1024. - PubMed
-
- Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level _ or _4.0 ng per milliliter. N Engl J Med. 2004;350:2239–2246. - PubMed
-
- Catalona WJ, Smith DS, Ratliff TL, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991;324:1156–1161. - PubMed
-
- Tricoli JV, Schoenfeldt M, Conley BA. Detection of prostate cancer and predicting progression: Current and future diagnostic markers. Clin Can Res. 2004;10:3943–3953. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

