MUC1-C oncoprotein functions as a direct activator of the nuclear factor-kappaB p65 transcription factor
- PMID: 19706766
- PMCID: PMC2760979
- DOI: 10.1158/0008-5472.CAN-09-0523
MUC1-C oncoprotein functions as a direct activator of the nuclear factor-kappaB p65 transcription factor
Abstract
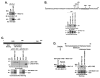
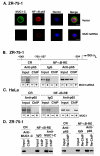
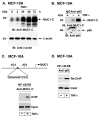
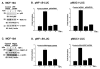
Nuclear factor-kappaB (NF-kappaB) is constitutively activated in diverse human malignancies. The mucin 1 (MUC1) oncoprotein is overexpressed in human carcinomas and, like NF-kappaB, blocks cell death and induces transformation. The present studies show that MUC1 constitutively associates with NF-kappaB p65 in carcinoma cells. The MUC1 COOH-terminal subunit (MUC1-C) cytoplasmic domain binds directly to NF-kappaB p65 and, importantly, blocks the interaction between NF-kappaB p65 and its inhibitor IkappaBalpha. We show that NF-kappaB p65 and MUC1-C constitutively occupy the promoter of the Bcl-xL gene in carcinoma cells and that MUC1-C contributes to NF-kappaB-mediated transcriptional activation. Studies in nonmalignant epithelial cells show that MUC1-C interacts with NF-kappaB in the response to tumor necrosis factor-alpha stimulation. Moreover, tumor necrosis factor-alpha induces the recruitment of NF-kappaB p65-MUC1-C complexes to NF-kappaB target genes, including the promoter of the MUC1 gene itself. We also show that an inhibitor of MUC1-C oligomerization blocks the interaction with NF-kappaB p65 in vitro and in cells. The MUC1-C inhibitor decreases MUC1-C and NF-kappaB p65 promoter occupancy and expression of NF-kappaB target genes. These findings indicate that MUC1-C is a direct activator of NF-kappaB p65 and that an inhibitor of MUC1 function is effective in blocking activation of the NF-kappaB pathway.
Figures

References
-
- Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–62. - PubMed
-
- Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002;3:221–7. - PubMed
-
- Hoffman A, Natoli G, Ghosh G. Transcriptional regulation via the NF-kappaB signaling module. Oncogene. 2006;25:6706–16. - PubMed
-
- Chen FE, Ghosh G. Regulation of DNA binding by Rel/NF-kappaB transcription factors: structural views. Oncogene. 1999;18:6845–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

