Divalent metal transporter 1 (DMT1) regulation by Ndfip1 prevents metal toxicity in human neurons
- PMID: 19706893
- PMCID: PMC2741278
- DOI: 10.1073/pnas.0904880106
Divalent metal transporter 1 (DMT1) regulation by Ndfip1 prevents metal toxicity in human neurons
Abstract
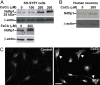
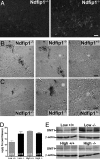
The regulation of metal ion transport within neurons is critical for normal brain function. Of particular importance is the regulation of redox metals such as iron (Fe), where excess levels can contribute to oxidative stress and protein aggregation, leading to neuronal death. The divalent metal transporter 1 (DMT1) plays a central role in the regulation of Fe as well as other metals; hence, failure of DMT1 regulation is linked to human brain pathology. However, it remains unclear how DMT1 is regulated in the brain. Here, we show that DMT1 is regulated by Ndfip1 (Nedd4 family-interacting protein 1), an adaptor protein that recruits E3 ligases to ubiquitinate target proteins. Using human neurons we show the Ndfip1 is upregulated and binds to DMT1 in response to Fe and cobalt (Co) exposure. This interaction results in the ubiquitination and degradation of DMT1, resulting in reduced metal entry. Induction of Ndfip1 expression protects neurons from metal toxicity, and removal of Ndfip1 by shRNAi results in hypersensitivity to metals. We identify Nedd4-2 as an E3 ligase recruited by Ndfip1 for the ubiquitination of DMT1 within human neurons. Comparison of brains from Ndfip1(-/-) with Ndfip1(+/+) mice exposed to Fe reveals that Ndfip1(-/-) brains accumulate Fe within neurons. Together, this evidence suggests a critical role for Ndfip1 in regulating metal transport in human neurons.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




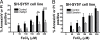

References
-
- Bush AI. Metals and neuroscience. Curr Opin Chem Biol. 2000;4:184–191. - PubMed
-
- Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR. Iron, brain ageing, and neurodegenerative disorders. Nat Rev Neurosci. 2004;5:863–873. - PubMed
-
- Halliwell B. Oxidative stress and neurodegeneration: Where are we now? J Neurochem. 2006;97:1634–1658. - PubMed
-
- Valko M, Morris H, Cronin MT. Metals, toxicity, and oxidative stress. Curr Med Chem. 2005;12:1161–1208. - PubMed
-
- Taylor EM, Crowe A, Morgan EH. Transferrin and iron uptake by the brain: Effects of altered iron status. J Neurochem. 1991;57:1584–1592. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

