VEGF(121)b, a new member of the VEGF(xxx)b family of VEGF-A splice isoforms, inhibits neovascularisation and tumour growth in vivo
- PMID: 19707198
- PMCID: PMC2768092
- DOI: 10.1038/sj.bjc.6605249
VEGF(121)b, a new member of the VEGF(xxx)b family of VEGF-A splice isoforms, inhibits neovascularisation and tumour growth in vivo
Abstract
Background: The key mediator of new vessel formation in cancer and other diseases is VEGF-A. VEGF-A exists as alternatively spliced isoforms - the pro-angiogenic VEGF(xxx) family generated by exon 8 proximal splicing, and a sister family, termed VEGF(xxx)b, exemplified by VEGF(165)b, generated by distal splicing of exon 8. However, it is unknown whether this anti-angiogenic property of VEGF(165)b is a general property of the VEGF(xxx)b family of isoforms.
Methods: The mRNA and protein expression of VEGF(121)b was studied in human tissue. The effect of VEGF(121)b was analysed by saturation binding to VEGF receptors, endothelial migration, apoptosis, xenograft tumour growth, pre-retinal neovascularisation and imaging of biodistribution in tumour-bearing mice with radioactive VEGF(121)b.
Results: The existence of VEGF(121)b was confirmed in normal human tissues. VEGF(121)b binds both VEGF receptors with similar affinity as other VEGF isoforms, but inhibits endothelial cell migration and is cytoprotective to endothelial cells through VEGFR-2 activation. Administration of VEGF(121)b normalised retinal vasculature by reducing both angiogenesis and ischaemia. VEGF(121)b reduced the growth of xenografted human colon tumours in association with reduced microvascular density, and an intravenous bolus of VEGF(121)b is taken up into colon tumour xenografts.
Conclusion: Here we identify a second member of the family, VEGF(121)b, with similar properties to those of VEGF(165)b, and underline the importance of the six amino acids of exon 8b in the anti-angiogenic activity of the VEGF(xxx)b isoforms.
Figures
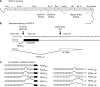


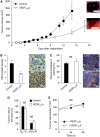
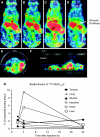

References
-
- Artac RA, McFee RM, Longfellow Smith RA, Baltes-Breitwisch MM, Clopton DT, Cupp AS (in press) Neutralization of vascular endothelial growth factor inhibitory isoforms is more effective than treatment with angiogenic isoforms in stimulating vascular development and follicle progression in the perinatal rat ovary. Biol Reprod - PMC - PubMed
-
- Bates DO, Cui TG, Doughty JM, Winkler M, Sugiono M, Shields JD, Peat D, Gillatt D, Harper SJ (2002) VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res 62: 4123–4131 - PubMed
-
- Bates DO, MacMillan PP, Manjaly JG, Qiu Y, Hudson SJ, Bevan HS, Hunter AJ, Soothill PW, Read M, Donaldson LF, Harper SJ (2006) The endogenous anti-angiogenic family of splice variants of VEGF, VEGFxxxb, are down-regulated in pre-eclamptic placentae at term. Clin Sci (Lond) 110: 575–585 - PubMed
-
- Cebe-Suarez S, Grunewald FS, Jaussi R, Li X, Claesson-Welsh L, Spillmann D, Mercer AA, Prota AE, Ballmer-Hofer K (2008) Orf virus VEGF-E NZ2 promotes paracellular NRP-1/VEGFR-2 coreceptor assembly via the peptide RPPR. FASEB J 22: 3078–3086 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

