Randomized phase II clinical trial of chemo-immunotherapy in advanced nonsmall cell lung cancer
- PMID: 19707385
- PMCID: PMC2721394
- DOI: 10.2147/btt.s2685
Randomized phase II clinical trial of chemo-immunotherapy in advanced nonsmall cell lung cancer
Abstract
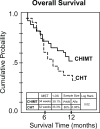
The purpose of this study was to compare chemotherapy-naive patients with stage IV nonsmall cell lung cancer patients treated with chemotherapy or chemoimmunotherapy. We tested doxetacel plus cisplatinum as chemotherapy protocol. An immunomodulatory adjuvant system was added as chemoimmunotherapy to the previously mentioned protocol. This system contains three well-known and complementary conditioners of protective immune-responses: cyclophosphamide low-dose, granulocyte macrophage-colony stimulant factor and magnesium silicate granuloma. Eighty-eight patients were randomly assigned to receive every 3-weeks one of the treatments under comparison. Patients received four cycles of treatment unless disease progression or unacceptable toxicity was documented. The maximum follow-up was one year. In each arm, tumor response (rate,duration), median survival time, 1-year overall survival, safety, and immunity modifications were assessed. Immunity was evaluated by submitting peripheral blood mononuclear cells to laboratory tests for nonspecific immunity: a) phytohemaglutinin-induced lymphocyte proliferation, b) prevalence of T-Regulatory (CD4+CD25+) cells and for specific immunity: a) lymphocyte proliferation induced by tumor-associated antigens (TAA) contained in a previously described autologous thermostable hemoderivative. The difference (chemotherapy vs. chemoimmunotherapy) in response rate induced by the two treatments (39.0% and 35.0%) was not statistically significant. However, the response duration (22 and 31 weeks), the median survival time (32 and 44 weeks) and 1-year survival (33.3% and 39.1%) were statistically higher with chemoimmunotherapy. No difference in toxicity between both arms was demonstrated. A switch in the laboratory immunity profile, nonspecific and specific, was associated with the chemoimmunotherapy treatment: increase of proliferative lymphocyte response, decrease of tolerogenic T-regulatory cells and eliciting TAA-sensitization.
Keywords: cancer therapy; cancer vaccine; immunomodulatory cancer treatment; immunotherapy adjuvants; lung cancer chemoimmunotherapy; lung cancer chemotherapy.
Figures
Similar articles
-
Adjuvant therapeutic vaccination in patients with non-small cell lung cancer made lymphopenic and reconstituted with autologous PBMC: first clinical experience and evidence of an immune response.J Transl Med. 2007 Sep 14;5:43. doi: 10.1186/1479-5876-5-43. J Transl Med. 2007. PMID: 17868452 Free PMC article. Clinical Trial.
-
Immune modulations during chemoimmunotherapy & novel vaccine strategies--in metastatic melanoma and non small-cell lung cancer.Dan Med J. 2013 Dec;60(12):B4774. Dan Med J. 2013. PMID: 24355457 Review.
-
Phase I/II combined chemoimmunotherapy with carcinoembryonic antigen-derived HLA-A2-restricted CAP-1 peptide and irinotecan, 5-fluorouracil, and leucovorin in patients with primary metastatic colorectal cancer.Clin Cancer Res. 2005 Aug 15;11(16):5993-6001. doi: 10.1158/1078-0432.CCR-05-0018. Clin Cancer Res. 2005. PMID: 16115944 Clinical Trial.
-
Randomized controlled phase III trial of adjuvant chemoimmunotherapy with activated cytotoxic T cells and dendritic cells from regional lymph nodes of patients with lung cancer.Cancer Immunol Immunother. 2018 Aug;67(8):1231-1238. doi: 10.1007/s00262-018-2180-6. Epub 2018 May 31. Cancer Immunol Immunother. 2018. PMID: 29855695 Free PMC article. Clinical Trial.
-
Rituximab: a review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia.Drugs. 2003;63(8):803-43. doi: 10.2165/00003495-200363080-00005. Drugs. 2003. PMID: 12662126 Review.
Cited by
-
Addition of an induction regimen of antiangiogenesis and antitumor immunity to standard chemotherapy improves survival in advanced malignancies.Med Oncol. 2012 Dec;29(5):3626-33. doi: 10.1007/s12032-012-0301-1. Epub 2012 Jul 19. Med Oncol. 2012. PMID: 22810591 Free PMC article. Clinical Trial.
-
Strengths and weaknesses of immunotherapy for advanced non-small-cell lung cancer: a meta-analysis of 12 randomized controlled trials.PLoS One. 2012;7(3):e32695. doi: 10.1371/journal.pone.0032695. Epub 2012 Mar 5. PLoS One. 2012. PMID: 22403699 Free PMC article.
References
-
- Altman DG, Schulz KF, Moher D, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med. 2001;134:663–94. - PubMed
-
- [ALA] American Lung Association Epidemiology and statistical unit. Research and program services. Trends in lung cancer morbidity and mortality 2005.
-
- Berd D, Mastrangelo MJ, Engstrom PF, et al. Augmentation of the human immune response by cyclophosphamide. Can Res. 1982;42:4862–6. - PubMed
-
- Chretien PB, Lipson SD, Makuch RW, et al. Effects of thymosin in vitro in cancer patients and correlation with clinical course after thymosin immunotherapy. Ann N Y Acad Sci. 1979;332:135–47. - PubMed
-
- Cochran AJ, Huang RR, Lee J, et al. Tumour-induced immune modulation of sentinel lymph nodes. Nat Rev Immunol. 2006;6:659–70. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

