Safety and efficacy of enzyme replacement therapy in the nephropathy of Fabry disease
- PMID: 19707461
- PMCID: PMC2727881
- DOI: 10.2147/btt.s3770
Safety and efficacy of enzyme replacement therapy in the nephropathy of Fabry disease
Abstract
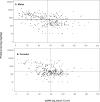
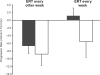
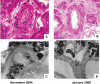
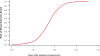
Kidney involvement with progressive loss of kidney function (Fabry nephropathy) is an important complication of Fabry disease, an X-linked lysosomal storage disorder arising from deficiency of alpha-galactosidase activity. Clinical trials have shown that enzyme replacement therapy (ERT) with recombinant human alpha-galactosidase clears globotriaosylceramide from kidney cells, and can stabilize kidney function in patients with mild to moderate Fabry nephropathy. Recent trials show that patients with more advanced Fabry nephropathy and overt proteinuria do not respond as well to ERT alone, but can benefit from anti-proteinuric therapy given in conjunction with ERT. This review focuses on the use of enzyme replacement therapy with agalsidase-alfa and agalsidase-beta in adults with Fabry nephropathy. The current results are reviewed and evaluated. The issues of dosing of enzyme replacement therapy, the use of adjunctive agents to control urinary protein excretion, and the individual factors that affect disease severity are reviewed.
Keywords: Fabry nephropathy; agalsidase; anti-proteinuric therapy; enzyme replacement therapy.
Figures






References
-
- Askari H, Kaneski CR, Semino-Mora C, et al. Cellular and tissue localization of globotriaosylceramide in Fabry disease. Virchows Arch. 2007;451:823–34. - PubMed
-
- Banikazemi M, Bultas J, Waldek S, et al. Agalsidase-beta therapy for advanced Fabry disease: a randomized trial. Ann Intern Med. 2007;146:77–86. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

