Neural stem/progenitor cells derived from the embryonic dorsal telencephalon of D6/GFP mice differentiate primarily into neurons after transplantation into a cortical lesion
- PMID: 19707869
- PMCID: PMC11498807
- DOI: 10.1007/s10571-009-9443-x
Neural stem/progenitor cells derived from the embryonic dorsal telencephalon of D6/GFP mice differentiate primarily into neurons after transplantation into a cortical lesion
Abstract
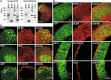
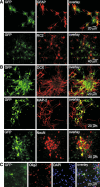
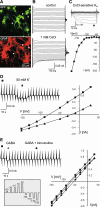
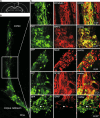
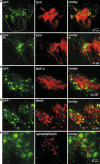
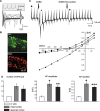
D6 is a promoter/enhancer of the mDach1 gene that is involved in the development of the neocortex and hippocampus. It is expressed by proliferating neural stem/progenitor cells (NSPCs) of the cortex at early stages of neurogenesis. The differentiation potential of NSPCs isolated from embryonic day 12 mouse embryos, in which the expression of green fluorescent protein (GFP) is driven by the D6 promoter/enhancer, has been studied in vitro and after transplantation into the intact adult rat brain as well as into the site of a photochemical lesion. The electrophysiological properties of D6/GFP-derived cells were studied using the whole-cell patch-clamp technique, and immunohistochemical analyses were carried out. D6/GFP-derived neurospheres expressed markers of radial glia and gave rise predominantly to immature neurons and GFAP-positive cells during in vitro differentiation. One week after transplantation into the intact brain or into the site of a photochemical lesion, transplanted cells expressed only neuronal markers. D6/GFP-derived neurons were characterised by the expression of tetrodotoxin-sensitive Na(+)-currents and K (A)- and K (DR) currents sensitive to 4-aminopyridine. They were able to fire repetitive action potentials and responded to the application of GABA. Our results indicate that after transplantation into the site of a photochemical lesion, D6/GFP-derived NSPCs survive and differentiate into neurons, and their membrane properties are comparable to those transplanted into the non-injured cortex. Therefore, region-specific D6/GFP-derived NSPCs represent a promising tool for studying neurogenesis and cell replacement in a damaged cellular environment.
Figures


Similar articles
-
Transplantation of embryonic neuroectodermal progenitor cells into the site of a photochemical lesion: immunohistochemical and electrophysiological analysis.J Neurobiol. 2006 Sep 1;66(10):1084-100. doi: 10.1002/neu.20278. J Neurobiol. 2006. PMID: 16838369
-
Forebrain-specific promoter/enhancer D6 derived from the mouse Dach1 gene controls expression in neural stem cells.Neuroscience. 2002;112(4):951-66. doi: 10.1016/s0306-4522(02)00053-2. Neuroscience. 2002. PMID: 12088753
-
Functional properties of neurons derived from fetal mouse neurospheres are compatible with those of neuronal precursors in vivo.J Neurosci Res. 2006 Jun;83(8):1494-501. doi: 10.1002/jnr.20835. J Neurosci Res. 2006. PMID: 16547970
-
A long, remarkable journey: tangential migration in the telencephalon.Nat Rev Neurosci. 2001 Nov;2(11):780-90. doi: 10.1038/35097509. Nat Rev Neurosci. 2001. PMID: 11715055 Review. No abstract available.
-
MicroRNAs in adult and embryonic neurogenesis.Neuromolecular Med. 2009;11(3):141-52. doi: 10.1007/s12017-009-8077-y. Epub 2009 Jul 14. Neuromolecular Med. 2009. PMID: 19598002 Free PMC article. Review.
Cited by
-
Highly efficient differentiation of neural precursors from human embryonic stem cells and benefits of transplantation after ischemic stroke in mice.Stem Cell Res Ther. 2013 Aug 8;4(4):93. doi: 10.1186/scrt292. Stem Cell Res Ther. 2013. PMID: 23928330 Free PMC article.
-
Heterogeneity of astrocytes: from development to injury - single cell gene expression.PLoS One. 2013 Aug 5;8(8):e69734. doi: 10.1371/journal.pone.0069734. Print 2013. PLoS One. 2013. PMID: 23940528 Free PMC article.
-
In vitro differentiation of mouse embryonic stem cells into neurons of the dorsal forebrain.Cell Mol Neurobiol. 2011 Jul;31(5):715-27. doi: 10.1007/s10571-011-9669-2. Epub 2011 Mar 20. Cell Mol Neurobiol. 2011. PMID: 21424551 Free PMC article.
References
-
- Abematsu M, Kagawa T, Fukuda S, Inoue T, Takebayashi H, Komiya S, Taga T (2006) Basic fibroblast growth factor endows dorsal telencephalic neural progenitors with the ability to differentiate into oligodendrocytes but not gamma-aminobutyric acidergic neurons. J Neurosci Res 83(5):731–743 - PubMed
-
- Akesson E, Piao JH, Samuelsson EB, Holmberg L, Kjaeldgaard A, Falci S, Sundstrom E, Seiger A (2007) Long-term culture and neuronal survival after intraspinal transplantation of human spinal cord-derived neurospheres. Physiol Behav 92(1–2):60–66 - PubMed
-
- Anderova M, Antonova T, Petrik D, Neprasova H, Chvatal A, Sykova E (2004) Voltage-dependent potassium currents in hypertrophied rat astrocytes after a cortical stab wound. Glia 48(4):311–326 - PubMed
-
- Anderova M, Kubinova S, Jelitai M, Neprasova H, Glogarova K, Prajerova I, Urdzikova L, Chvatal A, Sykova E (2006) Transplantation of embryonic neuroectodermal progenitor cells into the site of a photochemical lesion: immunohistochemical and electrophysiological analysis. J Neurobiol 66(10):1084–1100 - PubMed
-
- Andreasen M, Skov J, Nedergaard S (2007) Inwardly rectifying K(+) (Kir) channels antagonize ictal-like epileptiform activity in area CA1 of the rat hippocampus. Hippocampus 17(11):1037–1048 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

