The proteostasis boundary in misfolding diseases of membrane traffic
- PMID: 19708088
- PMCID: PMC2805282
- DOI: 10.1016/j.febslet.2009.07.014
The proteostasis boundary in misfolding diseases of membrane traffic
Abstract
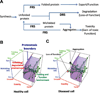
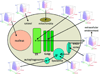
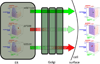
Protein function is regulated by the proteostasis network (PN) [Balch, W.E., Morimoto, R.I., Dillin, A. and Kelly, J.W. (2008) Adapting proteostasis for disease intervention. Science 319, 916-919], an integrated biological system that generates and protects the protein fold. The composition of the PN is regulated by signaling pathways including the unfolded protein response (UPR), the heat-shock response (HSR), the ubiquitin proteasome system (UPS) and epigenetic programs. Mismanagement of protein folding and function during membrane trafficking through the exocytic and endocytic pathways of eukaryotic cells by the PN is responsible for a wide range of diseases that include, among others, lysosomal storage diseases, myelination diseases, cystic fibrosis, systemic amyloidoses such as light chain myeloma, and neurodegenerative diseases including Alzheimer's. Toxicity from misfolding can be cell autonomous (affect the producing cell) or cell non-autonomous (affect a non-producing cell) or both, and have either a loss-of-function or gain-of-toxic function phenotype. Herein, we review the role of the PN and its regulatory transcriptional circuitry likely to be operational in managing the protein fold and function during membrane trafficking. We emphasize the enabling principle of a 'proteostasis boundary (PB)' [Powers, E.T., Morimoto, R.T., Dillin, A., Kelly, J.W., and Balch, W.E. (2009) Biochemical and chemical approaches to diseases of proteostasis deficiency. Annu. Rev. Biochem. 78, 959-991]. The PB is defined by the combined effects of the kinetics and thermodynamics of folding and the kinetics of misfolding, which are linked to the variable and adjustable PN capacity found different cell types. Differences in the PN account for the versatility of protein folding and function in health, and the cellular and tissue response to mutation and environmental challenges in disease. We discuss how manipulation of the folding energetics or the PB through metabolites and pharmacological intervention provides multiple routes for restoration of biological function in trafficking disease.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
- HL079442/HL/NHLBI NIH HHS/United States
- R01 DK075295/DK/NIDDK NIH HHS/United States
- R01 GM042336/GM/NIGMS NIH HHS/United States
- AG18917/AG/NIA NIH HHS/United States
- DK51870/DK/NIDDK NIH HHS/United States
- R01 HL095524/HL/NHLBI NIH HHS/United States
- R01 DK051870/DK/NIDDK NIH HHS/United States
- R01 GM033301/GM/NIGMS NIH HHS/United States
- R01 AG018917/AG/NIA NIH HHS/United States
- GM42336/GM/NIGMS NIH HHS/United States
- DK75295/DK/NIDDK NIH HHS/United States
- R01 HL079442/HL/NHLBI NIH HHS/United States
- GM33301/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

