Excited state proton transfer in the red fluorescent protein mKeima
- PMID: 19708654
- PMCID: PMC2754865
- DOI: 10.1021/ja904665x
Excited state proton transfer in the red fluorescent protein mKeima
Abstract
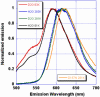
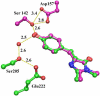
mKeima is an unusual monomeric red fluorescent protein (lambda(em)(max) approximately 620 nm) that is maximally excited in the blue (lambda(ex)(max) approximately 440 nm). The large Stokes shift suggests that the chromophore is normally protonated. A 1.63 A resolution structure of mKeima reveals the chromophore to be imbedded in a novel hydrogen bond network, different than in GFP, which could support proton transfer from the chromophore hydroxyl, via Ser142, to Asp157. At low temperatures the emission contains a green component (lambda(em)(max) approximately 535 nm), enhanced by deuterium substitution, presumably resulting from reduced proton transfer efficiency. Ultrafast pump/probe studies reveal a rising component in the 610 nm emission with a lifetime of approximately 4 ps, characterizing the rate of proton transfer. Mutation of Asp157 to neutral Asn changes the chromophore resting charge state to anionic (lambda(ex)(max) approximately 565 nm, lambda(em)(max) approximately 620 nm). Thus, excited state proton transfer (ESPT) explains the large Stokes shift. This work unambiguously characterizes green emission from the protonated acylimine chromophore of red fluorescent proteins.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

