Hippocampal replay of extended experience
- PMID: 19709631
- PMCID: PMC4364032
- DOI: 10.1016/j.neuron.2009.07.027
Hippocampal replay of extended experience
Abstract
During pauses in exploration, ensembles of place cells in the rat hippocampus re-express firing sequences corresponding to recent spatial experience. Such "replay" co-occurs with ripple events: short-lasting (approximately 50-120 ms), high-frequency (approximately 200 Hz) oscillations that are associated with increased hippocampal-cortical communication. In previous studies, rats exploring small environments showed replay anchored to the rat's current location and compressed in time into a single ripple event. Here, we show, using a neural decoding approach, that firing sequences corresponding to long runs through a large environment are replayed with high fidelity and that such replay can begin at remote locations on the track. Extended replay proceeds at a characteristic virtual speed of approximately 8 m/s and remains coherent across trains of ripple events. These results suggest that extended replay is composed of chains of shorter subsequences, which may reflect a strategy for the storage and flexible expression of memories of prolonged experience.
Figures
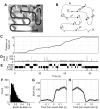



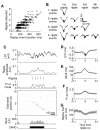
Comment in
-
Imagining the possibilities: ripples, routes, and reactivation.Neuron. 2009 Aug 27;63(4):421-3. doi: 10.1016/j.neuron.2009.08.002. Neuron. 2009. PMID: 19709624 Review.
References
-
- August DA, Levy WB. Temporal sequence compression by an integrate-and-fire model of hippocampal area CA3. J Comput Neurosci. 1999;6:71–90. - PubMed
-
- Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007;11:49–57. - PubMed
-
- Buzsaki G. Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience. 1989;31:551–570. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

