Lysophosphatidic acid-induced arterial wall remodeling: requirement of PPARgamma but not LPA1 or LPA2 GPCR
- PMID: 19709640
- PMCID: PMC2760670
- DOI: 10.1016/j.cellsig.2009.08.003
Lysophosphatidic acid-induced arterial wall remodeling: requirement of PPARgamma but not LPA1 or LPA2 GPCR
Abstract
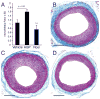
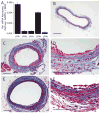
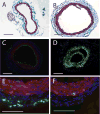
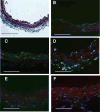
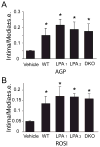
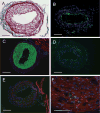
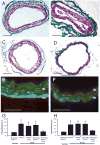
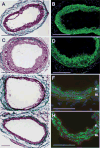
Lysophosphatidic acid (LPA) and its ether analog alkyl-glycerophosphate (AGP) elicit arterial wall remodeling when applied intralumenally into the uninjured carotid artery. LPA is the ligand of eight GPCRs and the peroxisome proliferator-activated receptor gamma (PPARgamma). We pursued a gene knockout strategy to identify the LPA receptor subtypes necessary for the neointimal response in a non-injury model of carotid remodeling and also compared the effects of AGP and the PPARgamma agonist rosiglitazone (ROSI) on balloon injury-elicited neointima development. In the balloon injury model AGP significantly increased neointima; however, rosiglitazone application attenuated it. AGP and ROSI were also applied intralumenally for 1h without injury into the carotid arteries of LPA(1), LPA(2), LPA(1&2) double knockout, and Mx1Cre-inducible conditional PPARgamma knockout mice targeted to vascular smooth muscle cells, macrophages, and endothelial cells. The neointima was quantified and also stained for CD31, CD68, CD11b, and alpha-smooth muscle actin markers. In LPA(1), LPA(2), LPA(1&2) GPCR knockout, Mx1Cre transgenic, PPARgamma(fl/-), and uninduced Mx1CrexPPARgamma(fl/-) mice AGP- and ROSI-elicited neointima was indistinguishable in its progression and cytological features from that of WT C57BL/6 mice. In PPARgamma(-/-) knockout mice, generated by activation of Mx1Cre-mediated recombination, AGP and ROSI failed to elicit neointima and vascular wall remodeling. Our findings point to a difference in the effects of AGP and ROSI between the balloon injury- and the non-injury chemically-induced neointima. The present data provide genetic evidence for the requirement of PPARgamma in AGP- and ROSI-elicited neointimal thickening in the non-injury model and reveal that the overwhelming majority of the cells in the neointimal layer express alpha-smooth muscle actin.
Figures
References
-
- Siess W, Tigyi G. J Cell Biochem. 2004;92(6):1086–1094. - PubMed
-
- Tsukahara T, Tsukahara R, Yasuda S, Makarova N, Valentine WJ, Allison P, Yuan H, Baker DL, Li Z, Bittman R, Parrill A, Tigyi G. J Biol Chem. 2006;281(6):3398–3407. - PubMed
-
- Xiao YJ, Schwartz B, Washington M, Kennedy A, Webster K, Belinson J, Xu Y. Anal Biochem. 2001;290(2):302–313. - PubMed
-
- Rother E, Brandl R, Baker DL, Goyal P, Gebhard H, Tigyi G, Siess W. Circulation. 2003;108(6):741–747. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

