POSH stimulates the ubiquitination and the clathrin-independent endocytosis of ROMK1 channels
- PMID: 19710010
- PMCID: PMC2785594
- DOI: 10.1074/jbc.M109.041582
POSH stimulates the ubiquitination and the clathrin-independent endocytosis of ROMK1 channels
Abstract
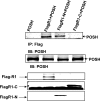
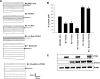
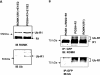
POSH (plenty of SH3) is a scaffold protein that has been shown to act as an E3 ubiquitin ligase. Here we report that POSH stimulates the ubiquitination of Kir1.1 (ROMK) and enhances the internalization of this potassium channel. Immunostaining reveals the expression of POSH in the renal cortical collecting duct. Immunoprecipitation of renal tissue lysate with ROMK antibody and glutathione S-transferase pulldown experiments demonstrated the association between ROMK and POSH. Moreover, immunoprecipitation of lysates of HEK293T cells transfected with ROMK1 or with constructs encoding the ROMK-N terminus or ROMK1-C-Terminus demonstrated that POSH binds to ROMK1 on its N terminus. To study the effect of POSH on ROMK1 channels, we measured potassium currents with electrophysiological methods in HEK293T cells and in oocytes transfected or injected with ROMK1 and POSH. POSH decreased potassium currents, and the inhibitory effect of POSH on ROMK channels was dose-dependent. Biotinylation assay further showed that POSH decreased surface expression of ROMK channels in HEK293T cells transfected with ROMK1 and POSH. The effect of POSH on ROMK1 channels was specific because POSH did not inhibit sodium current in oocytes injected with ENaC-alpha, beta, and gamma subunits. Moreover, POSH still decreased the potassium current in oocytes injected with a ROMK1 mutant (R1Delta373-378), in which a clathrin-dependent tyrosine-based internalization signal residing between amino acid residues 373 and 378 is deleted. However, the inhibitory effect of POSH on ROMK channels was absent in cells expressing with dominant negative dynamin and POSHDeltaRING, in which the RING domain was deleted. Expression of POSH also increased the ubiquitination of ROMK1, whereas expression of POSHDeltaRING diminished its ubiquitination in HEK293T cells. The notion that POSH may serve as an E3 ubiquitin ligase is also supported by in vitro ubiquitination assays in which adding POSH increased the ROMK ubiquitination. We conclude that POSH inhibits ROMK channels by enhancing dynamin-dependent and clathrin-independent endocytosis and by stimulating ubiquitination of ROMK channels.
Figures



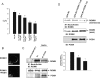
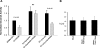

Similar articles
-
Expression of tetraspan protein CD63 activates protein-tyrosine kinase (PTK) and enhances the PTK-induced inhibition of ROMK channels.J Biol Chem. 2008 Mar 21;283(12):7674-81. doi: 10.1074/jbc.M705574200. Epub 2008 Jan 22. J Biol Chem. 2008. PMID: 18211905
-
Evidence for endocytosis of ROMK potassium channel via clathrin-coated vesicles.Am J Physiol Renal Physiol. 2002 Oct;283(4):F630-9. doi: 10.1152/ajprenal.00378.2001. Am J Physiol Renal Physiol. 2002. PMID: 12217853
-
MicroRNA 802 stimulates ROMK channels by suppressing caveolin-1.J Am Soc Nephrol. 2011 Jun;22(6):1087-98. doi: 10.1681/ASN.2010090927. Epub 2011 May 12. J Am Soc Nephrol. 2011. PMID: 21566059 Free PMC article.
-
Renal potassium channels: recent developments.Curr Opin Nephrol Hypertens. 2004 Sep;13(5):549-55. doi: 10.1097/00041552-200409000-00011. Curr Opin Nephrol Hypertens. 2004. PMID: 15300162 Review.
-
The protein tyrosine kinase-dependent pathway mediates the effect of K intake on renal K secretion.Physiology (Bethesda). 2005 Apr;20:140-6. doi: 10.1152/physiol.00044.2004. Physiology (Bethesda). 2005. PMID: 15772303 Review.
Cited by
-
Regulated endocytosis of NCC.Am J Physiol Renal Physiol. 2010 Aug;299(2):F297-9. doi: 10.1152/ajprenal.00280.2010. Epub 2010 May 26. Am J Physiol Renal Physiol. 2010. PMID: 20504880 Free PMC article. No abstract available.
-
The role of monoubiquitination in endocytic degradation of human ether-a-go-go-related gene (hERG) channels under low K+ conditions.J Biol Chem. 2011 Feb 25;286(8):6751-9. doi: 10.1074/jbc.M110.198408. Epub 2010 Dec 21. J Biol Chem. 2011. PMID: 21177251 Free PMC article.
-
Ubiquitination mediates Kv1.3 endocytosis as a mechanism for protein kinase C-dependent modulation.Sci Rep. 2017 Feb 10;7:42395. doi: 10.1038/srep42395. Sci Rep. 2017. PMID: 28186199 Free PMC article.
-
Endocytosis: A Turnover Mechanism Controlling Ion Channel Function.Cells. 2020 Aug 4;9(8):1833. doi: 10.3390/cells9081833. Cells. 2020. PMID: 32759790 Free PMC article. Review.
-
Sorting it out in endosomes: an emerging concept in renal epithelial cell transport regulation.Physiology (Bethesda). 2010 Oct;25(5):280-92. doi: 10.1152/physiol.00022.2010. Physiology (Bethesda). 2010. PMID: 20940433 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

