Phosphorylation of threonine 3: implications for Huntingtin aggregation and neurotoxicity
- PMID: 19710014
- PMCID: PMC2785575
- DOI: 10.1074/jbc.M109.013193
Phosphorylation of threonine 3: implications for Huntingtin aggregation and neurotoxicity
Abstract
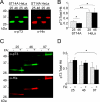
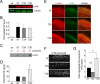
Huntingtin (Htt) is a widely expressed protein that causes tissue-specific degeneration when mutated to contain an expanded polyglutamine (poly(Q)) domain. Although Htt is large, 350 kDa, the appearance of amino-terminal fragments of Htt in extracts of postmortem brain tissue from patients with Huntington disease (HD), and the fact that an amino-terminal fragment, Htt exon 1 protein (Httex1p), is sufficient to cause disease in models of HD, points to the importance of the amino-terminal region of Htt in the disease process. The first exon of Htt encodes 17 amino acids followed by a poly(Q) repeat of variable length and culminating with a proline-rich domain of 50 amino acids. Because modifications to this fragment have the potential to directly affect pathogenesis in several ways, we have surveyed this fragment for potential post-translational modifications that might affect Htt behavior and detected several modifications of Httex1p. Here we report that the most prevalent modifications of Httex1p are NH(2)-terminal acetylation and phosphorylation of threonine 3 (pThr-3). We demonstrate that pThr-3 occurs on full-length Htt in vivo, and that this modification affects the aggregation and pathogenic properties of Htt. Thus, therapeutic strategies that modulate these events could in turn affect Htt pathogenesis.
Figures
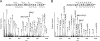


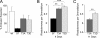
References
-
- The Huntington's Disease Collaborative Research Group (1993) Cell 72, 971–983 - PubMed
-
- Davies S. W., Turmaine M., Cozens B. A., DiFiglia M., Sharp A. H., Ross C. A., Scherzinger E., Wanker E. E., Mangiarini L., Bates G. P. (1997) Cell 90, 537–548 - PubMed
-
- Steffan J. S., Bodai L., Pallos J., Poelman M., McCampbell A., Apostol B. L., Kazantsev A., Schmidt E., Zhu Y. Z., Greenwald M., Kurokawa R., Housman D. E., Jackson G. R., Marsh J. L., Thompson L. M. (2001) Nature 413, 739–743 - PubMed
-
- Mangiarini L., Sathasivam K., Seller M., Cozens B., Harper A., Hetherington C., Lawton M., Trottier Y., Lehrach H., Davies S. W., Bates G. P. (1996) Cell 87, 493–506 - PubMed
-
- DiFiglia M., Sapp E., Chase K. O., Davies S. W., Bates G. P., Vonsattel J. P., Aronin N. (1997) Science 277, 1990–1993 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

