A novel class of cyclin-dependent kinase inhibitors identified by molecular docking act through a unique mechanism
- PMID: 19710018
- PMCID: PMC2785623
- DOI: 10.1074/jbc.M109.055251
A novel class of cyclin-dependent kinase inhibitors identified by molecular docking act through a unique mechanism
Abstract
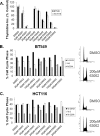
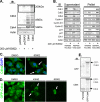
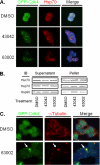
The cyclin-dependent kinase (Cdk) family is emerging as an important therapeutic target in the treatment of cancer. Cdks 1, 2, 4, and 6 are the key members that regulate the cell cycle, as opposed to Cdks that control processes such as transcription (Cdk7 and Cdk9). For this reason, Cdks 1, 2, 4, and 6 have been the subject of extensive cell cycle-related research, and consequently many inhibitors have been developed to target these proteins. However, the compounds that comprise the current list of Cdk inhibitors are largely ATP-competitive. Here we report the identification of a novel structural site on Cdk2, which is well conserved between the cell cycle Cdks. Small molecules identified by a high throughput in silico screen of this pocket exhibit cytostatic effects and act by reducing the apparent protein levels of cell cycle Cdks. Drug-induced cell cycle arrest is associated with decreased Rb phosphorylation and decreased expression of E2F-dependent genes. Multiple lines of evidence indicate that the primary mechanism of action of these compounds is the direct induction of Cdk1, Cdk2, and Cdk4 protein aggregation.
Figures




Similar articles
-
Cyclin-dependent protein kinase inhibitors including palbociclib as anticancer drugs.Pharmacol Res. 2016 May;107:249-275. doi: 10.1016/j.phrs.2016.03.012. Epub 2016 Mar 16. Pharmacol Res. 2016. PMID: 26995305 Review.
-
Cyclin-dependent protein serine/threonine kinase inhibitors as anticancer drugs.Pharmacol Res. 2019 Jan;139:471-488. doi: 10.1016/j.phrs.2018.11.035. Epub 2018 Nov 30. Pharmacol Res. 2019. PMID: 30508677 Review.
-
In vitro antitumor properties of a novel cyclin-dependent kinase inhibitor, P276-00.Mol Cancer Ther. 2007 Mar;6(3):918-25. doi: 10.1158/1535-7163.MCT-06-0613. Mol Cancer Ther. 2007. PMID: 17363486
-
The development of a selective cyclin-dependent kinase inhibitor that shows antitumor activity.Cancer Res. 2009 Aug 1;69(15):6208-15. doi: 10.1158/0008-5472.CAN-09-0301. Epub 2009 Jul 28. Cancer Res. 2009. PMID: 19638587 Free PMC article.
-
Cell cycle exit during terminal erythroid differentiation is associated with accumulation of p27(Kip1) and inactivation of cdk2 kinase.Blood. 2000 Oct 15;96(8):2746-54. Blood. 2000. PMID: 11023508
Cited by
-
Signaling mechanisms that suppress the cytostatic actions of rapamycin.PLoS One. 2014 Jun 13;9(6):e99927. doi: 10.1371/journal.pone.0099927. eCollection 2014. PLoS One. 2014. PMID: 24927123 Free PMC article.
-
Repurposing Tranexamic Acid as an Anticancer Agent.Front Pharmacol. 2022 Jan 13;12:792600. doi: 10.3389/fphar.2021.792600. eCollection 2021. Front Pharmacol. 2022. PMID: 35095503 Free PMC article.
-
Structure-based discovery of the first allosteric inhibitors of cyclin-dependent kinase 2.Cell Cycle. 2014;13(14):2296-305. doi: 10.4161/cc.29295. Epub 2014 Jun 9. Cell Cycle. 2014. PMID: 24911186 Free PMC article.
-
Novel agents that downregulate EGFR, HER2, and HER3 in parallel.Oncotarget. 2015 Apr 30;6(12):10445-59. doi: 10.18632/oncotarget.3398. Oncotarget. 2015. PMID: 25865227 Free PMC article.
-
Cyclin-Dependent Kinase Inhibitors as Anticancer Therapeutics.Mol Pharmacol. 2015 Nov;88(5):846-52. doi: 10.1124/mol.115.099325. Epub 2015 May 27. Mol Pharmacol. 2015. PMID: 26018905 Free PMC article.
References
-
- Deshpande A., Sicinski P., Hinds P. W. (2005) Oncogene 24, 2909–2915 - PubMed
-
- Sánchez I., Dynlacht B. D. (2005) Semin. Cell Dev. Biol. 16, 311–321 - PubMed
-
- Christian B. A., Grever M. R., Byrd J. C., Lin T. S. (2007) Curr. Opin. Oncol. 19, 573–578 - PubMed
-
- Singh R. P., Deep G., Blouin M. J., Pollak M. N., Agarwal R. (2007) Carcinogenesis 28, 2567–2574 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

