Human immunodeficiency virus type 1 envelope gp120-induced partial T-cell receptor signaling creates an F-actin-depleted zone in the virological synapse
- PMID: 19710135
- PMCID: PMC2772796
- DOI: 10.1128/JVI.01440-09
Human immunodeficiency virus type 1 envelope gp120-induced partial T-cell receptor signaling creates an F-actin-depleted zone in the virological synapse
Abstract
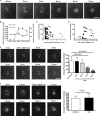
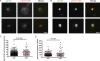
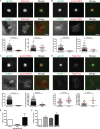
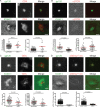
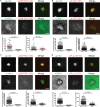
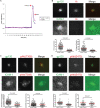
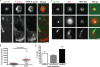
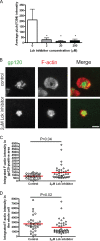
Cell-to-cell transmission of human immunodeficiency virus type 1 (HIV-1) occurs via a virological synapse (VS), a tight cell-cell junction formed between HIV-infected cells and target cells in which the HIV-1-infected cell polarizes and releases virions toward the noninfected target cell in a gp120- and intercellular adhesion molecule 1 (ICAM-1)-dependent process. The response of the target cell has been less studied. We utilized supported planar bilayers presenting gp120 and ICAM-1 as a reductionist model for the infected-cell membrane and investigated its effect on the target CD4 T cell. This study shows that HIV-1 gp120 interaction with its receptors is initially organized into microclusters that undergo F-actin-dependent consolidation into a central supramolecular activation complex (cSMAC). Src kinases are active in both gp120 microclusters and in the VS cSMAC. The early T-cell receptor (TCR) signaling machinery is partially activated at the VS, and signaling does not propagate to trigger Ca(2+) elevation or increase CD69 expression. However, these partial TCR signals act locally to create an F-actin-depleted zone. We propose a model in which the F-actin-depleted zone formed within the target CD4 T cell enhances the reception of virions by releasing the physical barrier for HIV-1 entry and facilitating postentry events.
Figures
Similar articles
-
Imaging of HIV-1 envelope-induced virological synapse and signaling on synthetic lipid bilayers.J Vis Exp. 2012 Mar 8;(61):3757. doi: 10.3791/3757. J Vis Exp. 2012. PMID: 22433250 Free PMC article.
-
HIV Envelope gp120 Alters T Cell Receptor Mobilization in the Immunological Synapse of Uninfected CD4 T Cells and Augments T Cell Activation.J Virol. 2016 Nov 14;90(23):10513-10526. doi: 10.1128/JVI.01532-16. Print 2016 Dec 1. J Virol. 2016. PMID: 27630246 Free PMC article.
-
Human immunodeficiency virus type 1 envelope gp120 induces a stop signal and virological synapse formation in noninfected CD4+ T cells.J Virol. 2008 Oct;82(19):9445-57. doi: 10.1128/JVI.00835-08. Epub 2008 Jul 16. J Virol. 2008. PMID: 18632854 Free PMC article.
-
Induction of lymphomonocyte activation by HIV-1 glycoprotein gp120. Possible role in AIDS pathogenesis.J Biol Regul Homeost Agents. 1996 Oct-Dec;10(4):83-91. J Biol Regul Homeost Agents. 1996. PMID: 9604776 Review.
-
Selected signalling proteins recruited to the T-cell receptor-CD3 complex.Immunology. 2018 Jan;153(1):42-50. doi: 10.1111/imm.12809. Epub 2017 Sep 5. Immunology. 2018. PMID: 28771705 Free PMC article. Review.
Cited by
-
The immunological synapse: the gateway to the HIV reservoir.Immunol Rev. 2013 Jul;254(1):305-25. doi: 10.1111/imr.12080. Immunol Rev. 2013. PMID: 23772628 Free PMC article. Review.
-
HIV: cell binding and entry.Cold Spring Harb Perspect Med. 2012 Aug 1;2(8):a006866. doi: 10.1101/cshperspect.a006866. Cold Spring Harb Perspect Med. 2012. PMID: 22908191 Free PMC article. Review.
-
The regulated secretory pathway in CD4(+) T cells contributes to human immunodeficiency virus type-1 cell-to-cell spread at the virological synapse.PLoS Pathog. 2011 Sep;7(9):e1002226. doi: 10.1371/journal.ppat.1002226. Epub 2011 Sep 1. PLoS Pathog. 2011. PMID: 21909273 Free PMC article.
-
HIV-Captured DCs Regulate T Cell Migration and Cell-Cell Contact Dynamics to Enhance Viral Spread.iScience. 2020 Aug 21;23(8):101427. doi: 10.1016/j.isci.2020.101427. Epub 2020 Aug 1. iScience. 2020. PMID: 32798973 Free PMC article.
-
Mechanisms for Cell-to-Cell Transmission of HIV-1.Front Immunol. 2018 Feb 19;9:260. doi: 10.3389/fimmu.2018.00260. eCollection 2018. Front Immunol. 2018. PMID: 29515578 Free PMC article. Review.
References
-
- Arthos, J., C. Cicala, E. Martinelli, K. Macleod, D. Van Ryk, D. Wei, Z. Xiao, T. D. Veenstra, T. P. Conrad, R. A. Lempicki, S. McLaughlin, M. Pascuccio, R. Gopaul, J. McNally, C. C. Cruz, N. Censoplano, E. Chung, K. N. Reitano, S. Kottilil, D. J. Goode, and A. S. Fauci. 2008. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat. Immunol. 9:301-309. - PubMed
-
- Barda-Saad, M., A. Braiman, R. Titerence, S. C. Bunnell, V. A. Barr, and L. E. Samelson. 2005. Dynamic molecular interactions linking the T cell antigen receptor to the actin cytoskeleton. Nat. Immunol. 6:80-89. - PubMed
-
- Barrero-Villar, M., J. R. Cabrero, M. Gordon-Alonso, J. Barroso-Gonzalez, S. Alvarez-Losada, M. A. Munoz-Fernandez, F. Sanchez-Madrid, and A. Valenzuela-Fernandez. 2009. Moesin is required for HIV-1-induced CD4-CXCR4 interaction, F-actin redistribution, membrane fusion and viral infection in lymphocytes. J. Cell Sci. 122:103-113. - PubMed
-
- Berg, L. J., L. D. Finkelstein, J. A. Lucas, and P. L. Schwartzberg. 2005. Tec family kinases in T lymphocyte development and function. Annu. Rev. Immunol. 23:549-600. - PubMed
-
- Bhaskar, P. T., and N. Hay. 2007. The two TORCs and Akt. Dev. Cell 12:487-502. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

