Mammalian orthoreovirus particles induce and are recruited into stress granules at early times postinfection
- PMID: 19710141
- PMCID: PMC2772771
- DOI: 10.1128/JVI.01239-09
Mammalian orthoreovirus particles induce and are recruited into stress granules at early times postinfection
Abstract
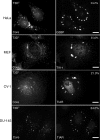
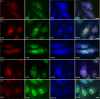
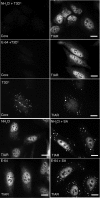
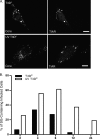
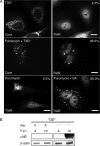
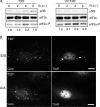
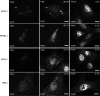
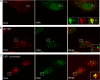
Infection with many mammalian orthoreovirus (MRV) strains results in shutoff of host, but not viral, protein synthesis via protein kinase R (PKR) activation and phosphorylation of translation initiation factor eIF2alpha. Following inhibition of protein synthesis, cellular mRNAs localize to discrete structures in the cytoplasm called stress granules (SGs), where they are held in a translationally inactive state. We examined MRV-infected cells to characterize SG formation in response to MRV infection. We found that SGs formed at early times following infection (2 to 6 h postinfection) in a manner dependent on phosphorylation of eIF2alpha. MRV induced SG formation in all four eIF2alpha kinase knockout cell lines, suggesting that at least two kinases are involved in induction of SGs. Inhibitors of MRV disassembly prevented MRV-induced SG formation, indicating that viral uncoating is a required step for SG formation. Neither inactivation of MRV virions by UV light nor treatment of MRV-infected cells with the translational inhibitor puromycin prevented SG formation, suggesting that viral transcription and translation are not required for SG formation. Viral cores were found to colocalize with SGs; however, cores from UV-inactivated virions did not associate with SGs, suggesting that viral core particles are recruited into SGs in a process that requires the synthesis of viral mRNA. These results demonstrate that MRV particles induce SGs in a step following viral disassembly but preceding viral mRNA transcription and that core particles are themselves recruited to SGs, suggesting that the cellular stress response may play a role in the MRV replication cycle.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

