The Epstein-Barr virus protein kinase BGLF4 and the exonuclease BGLF5 have opposite effects on the regulation of viral protein production
- PMID: 19710145
- PMCID: PMC2772808
- DOI: 10.1128/JVI.00525-09
The Epstein-Barr virus protein kinase BGLF4 and the exonuclease BGLF5 have opposite effects on the regulation of viral protein production
Abstract
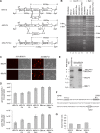
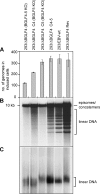
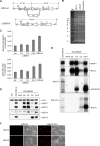
The Epstein-Barr virus BGLF4 and BGLF5 genes encode a protein kinase and an alkaline exonuclease, respectively. Both proteins were previously found to regulate multiple steps of virus replication, including lytic DNA replication and primary egress. However, while inactivation of BGLF4 led to the downregulation of several viral proteins, the absence of BGLF5 had the opposite effect. Using recombinant viruses that lack both viral enzymes, we confirm and extend these initial observations, e.g., by showing that both BGLF4 and BGLF5 are required for proper phosphorylation of the DNA polymerase processivity factor BMRF1. We further found that neither BGLF4 nor BGLF5 is required for baseline viral protein production. Complementation with BGLF5 downregulated mRNA levels and translation of numerous viral genes, though to various degrees, whereas BGLF4 had the opposite effect. BGLF4 and BGLF5 influences on viral expression were most pronounced for BFRF1 and BFLF2, two proteins essential for nuclear egress. For most viral genes studied, cotransfection of BGLF4 and BGLF5 had only a marginal influence on their expression patterns, showing that BGLF4 antagonizes BGLF5-mediated viral gene shutoff. To be able to exert its functions on viral gene expression, BGLF4 must be able to escape BGLF5's shutoff activities. Indeed, we found that BGLF5 stimulated the BGLF4 gene's transcription through an as yet uncharacterized molecular mechanism. The BGLF4/BGLF5 enzyme pair builds a regulatory loop that allows fine-tuning of virus protein production, which is required for efficient viral replication.
Figures




Similar articles
-
The Epstein-Barr virus alkaline exonuclease BGLF5 serves pleiotropic functions in virus replication.J Virol. 2009 May;83(10):4952-62. doi: 10.1128/JVI.00170-09. Epub 2009 Mar 4. J Virol. 2009. PMID: 19264771 Free PMC article.
-
The "Bridge" in the Epstein-Barr virus alkaline exonuclease protein BGLF5 contributes to shutoff activity during productive infection.J Virol. 2012 Sep;86(17):9175-87. doi: 10.1128/JVI.00309-12. Epub 2012 Jun 13. J Virol. 2012. PMID: 22696660 Free PMC article.
-
Efficient production of infectious viruses requires enzymatic activity of Epstein-Barr virus protein kinase.Virology. 2009 Jun 20;389(1-2):75-81. doi: 10.1016/j.virol.2009.04.007. Epub 2009 May 8. Virology. 2009. PMID: 19427010
-
Conquering the Nuclear Envelope Barriers by EBV Lytic Replication.Viruses. 2021 Apr 18;13(4):702. doi: 10.3390/v13040702. Viruses. 2021. PMID: 33919628 Free PMC article. Review.
-
Epstein-Barr virus evasion of CD8(+) and CD4(+) T cell immunity via concerted actions of multiple gene products.Semin Cancer Biol. 2008 Dec;18(6):397-408. doi: 10.1016/j.semcancer.2008.10.008. Epub 2008 Oct 25. Semin Cancer Biol. 2008. PMID: 18977445 Review.
Cited by
-
Conserved Herpesvirus Protein Kinases Target SAMHD1 to Facilitate Virus Replication.Cell Rep. 2019 Jul 9;28(2):449-459.e5. doi: 10.1016/j.celrep.2019.04.020. Cell Rep. 2019. PMID: 31291580 Free PMC article.
-
PIAS1 potentiates the anti-EBV activity of SAMHD1 through SUMOylation.Cell Biosci. 2021 Jul 8;11(1):127. doi: 10.1186/s13578-021-00636-y. Cell Biosci. 2021. PMID: 34238351 Free PMC article.
-
Viral serine/threonine protein kinases.J Virol. 2011 Feb;85(3):1158-73. doi: 10.1128/JVI.01369-10. Epub 2010 Nov 17. J Virol. 2011. PMID: 21084474 Free PMC article. Review.
-
Fine-tuning a blunt tool: Regulation of viral host shutoff RNases.PLoS Pathog. 2020 Apr 8;16(4):e1008385. doi: 10.1371/journal.ppat.1008385. eCollection 2020 Apr. PLoS Pathog. 2020. PMID: 32267905 Free PMC article. No abstract available.
-
Herpesvirus Late Gene Expression: A Viral-Specific Pre-initiation Complex Is Key.Front Microbiol. 2016 Jun 6;7:869. doi: 10.3389/fmicb.2016.00869. eCollection 2016. Front Microbiol. 2016. PMID: 27375590 Free PMC article. Review.
References
-
- Asai, R., A. Kato, K. Kato, M. Kanamori-Koyama, K. Sugimoto, T. Sairenji, Y. Nishiyama, and Y. Kawaguchi. 2006. Epstein-Barr virus protein kinase BGLF4 is a virion tegument protein that dissociates from virions in a phosphorylation-dependent process and phosphorylates the viral immediate-early protein BZLF1. J. Virol. 80:5125-5134. - PMC - PubMed
-
- Baer, R., A. T. Bankier, M. D. Biggin, P. L. Deininger, P. J. Farrell, T. J. Gibson, G. Hatfull, G. S. Hudson, S. C. Satchwell, C. Seguin, P. S. Tufnell, and B. G. Barell. 1984. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature 310:207-211. - PubMed
-
- Chen, H. F., M. Sauter, P. Haiss, and N. Muller-Lantzsch. 1991. Immunological characterization of the Epstein-Barr virus phosphoprotein PP58 and deoxyribonuclease expressed in the baculovirus expression system. Int. J. Cancer 48:879-888. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

