Fine-tuning of secondary arbor development: the effects of the ecdysone receptor on the adult neuronal lineages of the Drosophila thoracic CNS
- PMID: 19710167
- PMCID: PMC2739142
- DOI: 10.1242/dev.039859
Fine-tuning of secondary arbor development: the effects of the ecdysone receptor on the adult neuronal lineages of the Drosophila thoracic CNS
Abstract
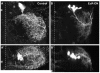
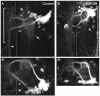
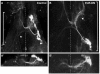
The adult central nervous system (CNS) of Drosophila is largely composed of relatively homogenous neuronal classes born during larval life. These adult-specific neuron lineages send out initial projections and then arrest development until metamorphosis, when intense sprouting occurs to establish the massive synaptic connections necessary for the behavior and function of the adult fly. In this study, we identified and characterized specific lineages in the adult CNS and described their secondary branch patterns. Because prior studies show that the outgrowth of incumbent remodeling neurons in the CNS is highly dependent on the ecdysone pathway, we investigated the role of ecdysone in the development of the adult-specific neuronal lineages using a dominant-negative construct of the ecdysone receptor (EcR-DN). When EcR-DN was expressed in clones of the adult-specific lineages, neuroblasts persisted longer, but we saw no alteration in the initial projections of the lineages. Defects were observed in secondary arbors of adult neurons, including clumping and cohesion of fine branches, misrouting, smaller arbors and some defasciculation. The defects varied across the multiple neuron lineages in both appearance and severity. These results indicate that the ecdysone receptor complex influences the fine-tuning of connectivity between neuronal circuits, in conjunction with other factors driving outgrowth and synaptic partnering.
Figures

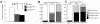

Similar articles
-
Two-factor specification of apoptosis: TGF-β signaling acts cooperatively with ecdysone signaling to induce cell- and stage-specific apoptosis of larval neurons during metamorphosis in Drosophila melanogaster.Apoptosis. 2019 Dec;24(11-12):972-989. doi: 10.1007/s10495-019-01574-4. Apoptosis. 2019. PMID: 31641960
-
Use of time-lapse imaging and dominant negative receptors to dissect the steroid receptor control of neuronal remodeling in Drosophila.Development. 2006 Jan;133(2):275-85. doi: 10.1242/dev.02191. Epub 2005 Dec 14. Development. 2006. PMID: 16354717
-
Programmed cell death mechanisms of identifiable peptidergic neurons in Drosophila melanogaster.Development. 2006 Jun;133(11):2223-32. doi: 10.1242/dev.02376. Epub 2006 May 3. Development. 2006. PMID: 16672345
-
Ecdysone signaling in adult Drosophila melanogaster.J Insect Physiol. 2012 Mar;58(3):293-302. doi: 10.1016/j.jinsphys.2012.01.013. Epub 2012 Jan 28. J Insect Physiol. 2012. PMID: 22310011 Review.
-
Drosophila postembryonic nervous system development: a model for the endocrine control of development.Genetics. 2023 Mar 2;223(3):iyac184. doi: 10.1093/genetics/iyac184. Genetics. 2023. PMID: 36645270 Free PMC article. Review.
Cited by
-
Temporal patterns of broad isoform expression during the development of neuronal lineages in Drosophila.Neural Dev. 2009 Nov 2;4:39. doi: 10.1186/1749-8104-4-39. Neural Dev. 2009. PMID: 19883497 Free PMC article.
-
Survival motor neuron protein regulates stem cell division, proliferation, and differentiation in Drosophila.PLoS Genet. 2011 Apr;7(4):e1002030. doi: 10.1371/journal.pgen.1002030. Epub 2011 Apr 7. PLoS Genet. 2011. PMID: 21490958 Free PMC article.
-
Dual role for Hox genes and Hox co-factors in conferring leg motoneuron survival and identity in Drosophila.Development. 2013 May;140(9):2027-38. doi: 10.1242/dev.090902. Epub 2013 Mar 27. Development. 2013. PMID: 23536569 Free PMC article.
-
Role of Notch signaling in establishing the hemilineages of secondary neurons in Drosophila melanogaster.Development. 2010 Jan;137(1):53-61. doi: 10.1242/dev.041749. Development. 2010. PMID: 20023160 Free PMC article.
-
Developmentally Arrested Precursors of Pontine Neurons Establish an Embryonic Blueprint of the Drosophila Central Complex.Curr Biol. 2019 Feb 4;29(3):412-425.e3. doi: 10.1016/j.cub.2018.12.012. Epub 2019 Jan 17. Curr Biol. 2019. PMID: 30661802 Free PMC article.
References
-
- Bai, J., Uehara, Y. and Montell, D. J. (2000). Regulation of invasive cell behavior by taiman, a Drosophila protein related to AIB1, a steroid receptor coactivator amplified in breast cancer. Cell 103, 1047-1058. - PubMed
-
- Berreur, P., Porcheron, P., Moriniere, M., Berreur-Bonnenfant, J., Belinski-Deutsch, S., Busson, D. and Lamour-Audit, C. (1984). Ecdysteroids during the third larval instar in 1(3)ecd-1ts, a temperature-sensitive mutant of Drosophila melanogaster. Gen. Comp. Endocrinol. 54, 76-84. - PubMed
-
- Britton, J. S. and Edgar, B. A. (1998). Environmental control of the cell cycle in Drosophila: nutrition activates mitotic and endoreplicative cells by distinct mechanisms. Development 125, 2149-2158. - PubMed
-
- Brown, H. L., Cherbas, L., Cherbas, P. and Truman, J. W. (2006). Use of time-lapse imaging and dominant negative receptors to dissect the steroid receptor control of neuronal remodeling in Drosophila. Development 133, 275-285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

