The pharmacokinetic behavior of the soy isoflavone metabolite S-(-)equol and its diastereoisomer R-(+)equol in healthy adults determined by using stable-isotope-labeled tracers
- PMID: 19710188
- PMCID: PMC2744624
- DOI: 10.3945/ajcn.2009.27981
The pharmacokinetic behavior of the soy isoflavone metabolite S-(-)equol and its diastereoisomer R-(+)equol in healthy adults determined by using stable-isotope-labeled tracers
Abstract
Background: The nonsteroidal estrogen equol occurs as diastereoisomers, S-(-)equol and R-(+)equol, both of which have significant biological actions. S-(-)equol, the naturally occurring enantiomer produced by 20-30% of adults consuming soy foods, has selective affinity for estrogen receptor-beta, whereas both enantiomers modulate androgen action. Little is known about the pharmacokinetics of the diastereoisomers, despite current interest in developing equol as a nutraceutical or pharmaceutical agent.
Objective: The objective was to compare the pharmacokinetics of S-(-)equol and R-(+)equol by using [13C] stable-isotope-labeled tracers to facilitate the optimization of clinical studies aimed at evaluating the potential of these diastereoisomers in the prevention and treatment of estrogen- and androgen-dependent conditions.
Design: A randomized, crossover, open-label study in 12 healthy adults (6 men and 6 women) compared the plasma and urinary pharmacokinetics of orally administered enantiomeric pure forms of S-(-)[2-13C]equol, R-(+)[2-13C]equol, and the racemic mixture. Plasma and urinary [13C]R-equol and [13C]S-equol concentrations were measured by tandem mass spectrometry.
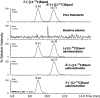
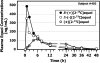
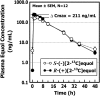
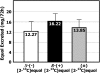
Results: Plasma [13C]equol concentration appearance and disappearance curves showed that both enantiomers were rapidly absorbed, attained high circulating concentrations, and had a similar terminal elimination half-life of 7-8 h. The systemic bioavailability and fractional absorption of R-(+)[2-13C]equol were higher than those of S-(-)[2-13C]equol or the racemate. The pharmacokinetics of racemic (+/-)[2-13C]equol were different from those of the individual enantiomers: slower absorption, lower peak plasma concentrations, and lower systemic bioavailability.
Conclusions: The high bioavailability of both diastereoisomers contrasts with previous findings for the soy isoflavones daidzein and genistein, both of which have relatively poor bioavailability, and suggests that low doses of equol taken twice daily may be sufficient to achieve biological effects.
Figures

References
-
- Setchell KDR, Borriello SP, Hulme P, Kirk DN, Axelson M. Nonsteroidal estrogens of dietary origin: possible roles in hormone-dependent disease. Am J Clin Nutr 1984;40:569–78 - PubMed
-
- Setchell KDR, Brown NM, Zimmer-Nechemias L, et al. Evidence for lack of absorption of soy isoflavone glycosides in humans, supporting the crucial role of intestinal metabolism for bioavailability. Am J Clin Nutr 2002;76:447–53 - PubMed
-
- Brown NM, Setchell KDR. Animal models impacted by phytoestrogens in commercial chow: implications for pathways influenced by hormones. Lab Invest 2001;81:735–47 - PubMed
-
- Thigpen JE, Haseman JK, Saunders HE, Setchell KDR, Grant MG, Forsythe DB. Dietary phytoestrogens accelerate the time of vaginal opening in immature CD-1 mice. Comp Med 2003;53:607–15 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

