Multimodal integration in rostral fastigial nucleus provides an estimate of body movement
- PMID: 19710303
- PMCID: PMC3311469
- DOI: 10.1523/JNEUROSCI.1937-09.2009
Multimodal integration in rostral fastigial nucleus provides an estimate of body movement
Abstract
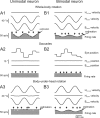
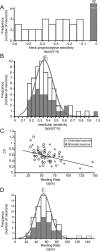
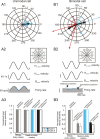
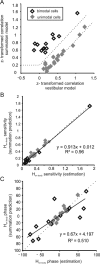
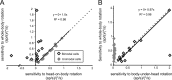
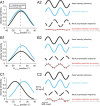
The ability to accurately control posture and perceive self-motion and spatial orientation requires knowledge of the motion of both the head and body. However, whereas the vestibular sensors and nuclei directly encode head motion, no sensors directly encode body motion. Instead, the convergence of vestibular and neck proprioceptive inputs during self-motion is generally believed to underlie the ability to compute body motion. Here, we provide evidence that the brain explicitly computes an internal estimate of body motion at the level of single cerebellar neurons. Neuronal responses were recorded from the rostral fastigial nucleus, the most medial of the deep cerebellar nuclei, during whole-body, body-under-head, and head-on-body rotations. We found that approximately half of the neurons encoded the motion of the body in space, whereas the other half encoded the motion of the head in space in a manner similar to neurons in the vestibular nuclei. Notably, neurons encoding body motion responded to both vestibular and proprioceptive stimulation (accordingly termed bimodal neurons). In contrast, neurons encoding head motion were sensitive only to vestibular inputs (accordingly termed unimodal neurons). Comparison of the proprioceptive and vestibular responses of bimodal neurons further revealed similar tuning in response to changes in head-on-body position. We propose that the similarity in nonlinear processing of vestibular and proprioceptive signals underlies the accurate computation of body motion. Furthermore, the same neurons that encode body motion (i.e., bimodal neurons) most likely encode vestibular signals in a body-referenced coordinate frame, since the integration of proprioceptive and vestibular information is required for both computations.
Figures



References
-
- Anastasopoulos D, Mergner T. Canal-neck interaction in vestibular nuclear neurons of the cat. Exp Brain Res. 1982;46:269–280. - PubMed
-
- Angelaki DE, Shaikh AG, Green AM, Dickman JD. Neurons compute internal models of the physical laws of motion. Nature. 2004;430:560–564. - PubMed
-
- Asanuma C, Thach WT, Jones EG. Distribution of cerebellar terminations and their relation to other afferent terminations in the ventral lateral thalamic region of the monkey. Brain Res. 1983;286:237–265. - PubMed
-
- Avillac M, Denève S, Olivier E, Pouget A, Duhamel JR. Reference frames for representing visual and tactile locations in parietal cortex. Nat Neurosci. 2005;8:941–949. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
