Downregulation of functional Reelin receptors in projection neurons implies that primary Reelin action occurs at early/premigratory stages
- PMID: 19710317
- PMCID: PMC6665715
- DOI: 10.1523/JNEUROSCI.0345-09.2009
Downregulation of functional Reelin receptors in projection neurons implies that primary Reelin action occurs at early/premigratory stages
Abstract
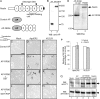
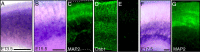
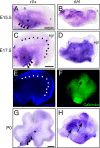
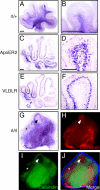
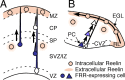
Reelin signaling is essential for correct development of the mammalian brain. Reelin binds to apolipoprotein E receptor 2 and very low-density lipoprotein receptor and induces phosphorylation of Dab1. However, when and where these reactions occur is essentially unknown, and the primary function(s) of Reelin remain unclear. Here, we used alkaline phosphatase fusion of the receptor-binding region of Reelin to quantitatively investigate the localization of functional Reelin receptors (i.e., those on the plasma membrane as mature forms) in the developing brain. In the wild-type cerebral cortex, they are mainly present in the intermediate and subventricular zones, as well as in radial fibers, but much less in the cell bodies of the cortical plate. Functional Reelin receptors are much more abundant in the Reelin-deficient cortical plate, indicating that Reelin induces their downregulation and that it begins before the neurons migrate out of the intermediate zone. In the wild-type cerebellum, functional Reelin receptors are mainly present in the cerebellar ventricular zone but scarcely expressed by Purkinje cells that have migrated out of it. It is thus strongly suggested that Reelin exerts critical actions on migrating projection neurons at their early/premigratory stages en route to their final destinations, in the developing cerebral cortex and cerebellum.
Figures


Similar articles
-
Reelin stabilizes the actin cytoskeleton of neuronal processes by inducing n-cofilin phosphorylation at serine3.J Neurosci. 2009 Jan 7;29(1):288-99. doi: 10.1523/JNEUROSCI.2934-08.2009. J Neurosci. 2009. PMID: 19129405 Free PMC article.
-
Migration, early axonogenesis, and Reelin-dependent layer-forming behavior of early/posterior-born Purkinje cells in the developing mouse lateral cerebellum.Neural Dev. 2010 Sep 1;5:23. doi: 10.1186/1749-8104-5-23. Neural Dev. 2010. PMID: 20809939 Free PMC article.
-
Ectopic Reelin induces neuronal aggregation with a normal birthdate-dependent "inside-out" alignment in the developing neocortex.J Neurosci. 2010 Aug 18;30(33):10953-66. doi: 10.1523/JNEUROSCI.0486-10.2010. J Neurosci. 2010. PMID: 20720102 Free PMC article.
-
A missed exit: Reelin sets in motion Dab1 polyubiquitination to put the break on neuronal migration.Genes Dev. 2007 Nov 15;21(22):2850-4. doi: 10.1101/gad.1622907. Genes Dev. 2007. PMID: 18006681 Review. No abstract available.
-
The reelin signaling pathway: some recent developments.Cereb Cortex. 2003 Jun;13(6):627-33. doi: 10.1093/cercor/13.6.627. Cereb Cortex. 2003. PMID: 12764038 Review.
Cited by
-
Dynamic FoxG1 expression coordinates the integration of multipolar pyramidal neuron precursors into the cortical plate.Neuron. 2012 Jun 21;74(6):1045-58. doi: 10.1016/j.neuron.2012.04.025. Neuron. 2012. PMID: 22726835 Free PMC article.
-
Physiological significance of proteolytic processing of Reelin revealed by cleavage-resistant Reelin knock-in mice.Sci Rep. 2020 Mar 11;10(1):4471. doi: 10.1038/s41598-020-61380-w. Sci Rep. 2020. PMID: 32161359 Free PMC article.
-
Importance of Reelin C-terminal region in the development and maintenance of the postnatal cerebral cortex and its regulation by specific proteolysis.J Neurosci. 2015 Mar 18;35(11):4776-87. doi: 10.1523/JNEUROSCI.4119-14.2015. J Neurosci. 2015. PMID: 25788693 Free PMC article.
-
N-cadherin-regulated FGFR ubiquitination and degradation control mammalian neocortical projection neuron migration.Elife. 2019 Oct 2;8:e47673. doi: 10.7554/eLife.47673. Elife. 2019. PMID: 31577229 Free PMC article.
-
Secreted Metalloproteinase ADAMTS-3 Inactivates Reelin.J Neurosci. 2017 Mar 22;37(12):3181-3191. doi: 10.1523/JNEUROSCI.3632-16.2017. Epub 2017 Feb 17. J Neurosci. 2017. PMID: 28213441 Free PMC article.
References
-
- Altman J, Bayer SA. Embryonic development of the rat cerebellum. II. Translocation and regional distribution of the deep neurons. J Comp Neurol. 1985;231:27–41. - PubMed
-
- Andersen OM, Benhayon D, Curran T, Willnow TE. Differential binding of ligands to the apolipoprotein E receptor 2. Biochemistry. 2003;42:9355–9364. - PubMed
-
- Benhayon D, Magdaleno S, Curran T. Binding of purified Reelin to ApoER2 and VLDLR mediates tyrosine phosphorylation of Disabled-1. Brain Res Mol Brain Res. 2003;112:33–45. - PubMed
-
- Cooper JA. A mechanism for inside-out lamination in the neocortex. Trends Neurosci. 2008;31:113–119. - PubMed
-
- D'Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
