Characterizing the appearance and growth of amyloid plaques in APP/PS1 mice
- PMID: 19710322
- PMCID: PMC2756291
- DOI: 10.1523/JNEUROSCI.2637-09.2009
Characterizing the appearance and growth of amyloid plaques in APP/PS1 mice
Abstract
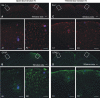
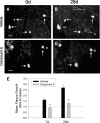
Amyloid plaques are primarily composed of extracellular aggregates of amyloid-beta (Abeta) peptide and are a pathological signature of Alzheimer's disease. However, the factors that influence the dynamics of amyloid plaque formation and growth in vivo are largely unknown. Using serial intravital multiphoton microscopy through a thinned-skull cranial window in APP/PS1 transgenic mice, we found that amyloid plaques appear and grow over a period of weeks before reaching a mature size. Growth was more prominent early after initial plaque formation: plaques grew faster in 6-month-old compared with 10-month-old mice. Plaque growth rate was also size-related, as smaller plaques exhibited more rapid growth relative to larger plaques. Alterations in interstitial Abeta concentrations were associated with changes in plaque growth. Parallel studies using multiphoton microscopy and in vivo microdialysis revealed that pharmacological reduction of soluble extracellular Abeta by as little as 20-25% was associated with a dramatic decrease in plaque formation and growth. Furthermore, this small reduction in Abeta synthesis was sufficient to reduce amyloid plaque load in 6-month-old but not 10-month-old mice, suggesting that treatment early in disease pathogenesis may be more effective than later treatment. In contrast to thinned-skull windows, no significant plaque growth was observed under open-skull windows, which demonstrated extensive microglial and astrocytic activation. Together, these findings indicate that individual amyloid plaque growth in vivo occurs over a period of weeks and may be influenced by interstitial Abeta concentration as well as reactive gliosis.
Figures




References
-
- Braakman N, Matysik J, van Duinen SG, Verbeek F, Schliebs R, de Groot HJ, Alia A. Longitudinal assessment of Alzheimer's β-amyloid plaque development in transgenic mice monitored by in vivo magnetic resonance microimaging. J Magn Reson Imaging. 2006;24:530–536. - PubMed
-
- Burdick D, Soreghan B, Kwon M, Kosmoski J, Knauer M, Henschen A, Yates J, Cotman C, Glabe C. Assembly and aggregation properties of synthetic Alzheimer's A4/β amyloid peptide analogs. J Biol Chem. 1992;267:546–554. - PubMed
-
- Busche MA, Eichhoff G, Adelsberger H, Abramowski D, Wiederhold KH, Haass C, Staufenbiel M, Konnerth A, Garaschuk O. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer's disease. Science. 2008;321:1686–1689. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
