Dynamic associations in the cerebellar-motoneuron network during motor learning
- PMID: 19710326
- PMCID: PMC6665687
- DOI: 10.1523/JNEUROSCI.2178-09.2009
Dynamic associations in the cerebellar-motoneuron network during motor learning
Abstract
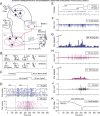
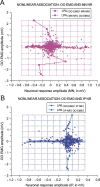
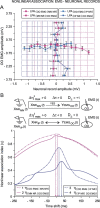
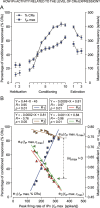
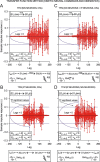
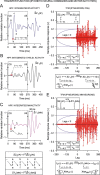
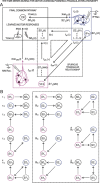
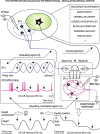
We assessed here true causal directionalities in cerebellar-motoneuron (MN) network associations during the classical conditioning of eyelid responses. For this, the firing activities of identified facial MNs and cerebellar interpositus (IP) nucleus neurons were recorded during the acquisition of this type of associative learning in alert behaving cats. Simultaneously, the eyelid conditioned response (CR) and the EMG activity of the orbicularis oculi (OO) muscle were recorded. Nonlinear association analysis and time-dependent causality method allowed us to determine the asymmetry, time delays, direction in coupling, and functional interdependences between neuronal recordings and learned motor responses. We concluded that the functional nonlinear association between the IP neurons and OO muscle activities was bidirectional and asymmetric, and the time delays in the two directions of coupling always lagged the start of the CR. Additionally, the strength of coupling depended inversely on the level of expression of eyeblink CRs, whereas causal inferences were significantly dependent on the phase information status. In contrast, the functional association between OO MNs and OO muscle activities was unidirectional and quasisymmetric, and the time delays in coupling were always of opposed signs. Moreover, information transfer in cerebellar-MN network associations during the learning process required a "driving common source" that induced the mere "modulating coupling" of the IP nucleus with the final common pathway for the eyelid motor system. Thus, it can be proposed that the cerebellum is always looking back and reevaluating its own function, using the information acquired in the process, to play a modulating-reinforcing role in motor learning.
Figures
Similar articles
-
Timing and causality in the generation of learned eyelid responses.Front Integr Neurosci. 2011 Aug 30;5:39. doi: 10.3389/fnint.2011.00039. eCollection 2011. Front Integr Neurosci. 2011. PMID: 21941469 Free PMC article.
-
Dynamic changes in the cerebellar-interpositus/red-nucleus-motoneuron pathway during motor learning.Cerebellum. 2011 Dec;10(4):702-10. doi: 10.1007/s12311-010-0242-1. Cerebellum. 2011. PMID: 21181461
-
The Motor Cortex Is Involved in the Generation of Classically Conditioned Eyelid Responses in Behaving Rabbits.J Neurosci. 2016 Jun 29;36(26):6988-7001. doi: 10.1523/JNEUROSCI.4190-15.2016. J Neurosci. 2016. PMID: 27358456 Free PMC article.
-
The role of interpositus nucleus in eyelid conditioned responses.Cerebellum. 2002 Dec;1(4):289-308. doi: 10.1080/147342202320883597. Cerebellum. 2002. PMID: 12879967 Review.
-
Firing activities of identified posterior interpositus nucleus neurons during associative learning in behaving cats.Brain Res Brain Res Rev. 2005 Sep;49(2):367-76. doi: 10.1016/j.brainresrev.2004.10.006. Brain Res Brain Res Rev. 2005. PMID: 16111563 Review.
Cited by
-
Properties of the nucleo-olivary pathway: an in vivo whole-cell patch clamp study.PLoS One. 2012;7(9):e46360. doi: 10.1371/journal.pone.0046360. Epub 2012 Sep 27. PLoS One. 2012. PMID: 23029495 Free PMC article.
-
Spike sorting based on shape, phase, and distribution features, and K-TOPS clustering with validity and error indices.Sci Rep. 2018 Dec 12;8(1):17796. doi: 10.1038/s41598-018-35491-4. Sci Rep. 2018. PMID: 30542106 Free PMC article.
-
Timing and causality in the generation of learned eyelid responses.Front Integr Neurosci. 2011 Aug 30;5:39. doi: 10.3389/fnint.2011.00039. eCollection 2011. Front Integr Neurosci. 2011. PMID: 21941469 Free PMC article.
-
Implicit Memory in Monkeys: Development of a Delay Eyeblink Conditioning System with Parallel Electromyographic and High-Speed Video Measurements.PLoS One. 2015 Jun 12;10(6):e0129828. doi: 10.1371/journal.pone.0129828. eCollection 2015. PLoS One. 2015. PMID: 26068663 Free PMC article.
-
Theta synchronization between medial prefrontal cortex and cerebellum is associated with adaptive performance of associative learning behavior.Sci Rep. 2016 Feb 16;6:20960. doi: 10.1038/srep20960. Sci Rep. 2016. PMID: 26879632 Free PMC article.
References
-
- Belsley DA, Kuh E, Welsch RE. Regression diagnostics: identifying influential data and sources of collinearity. New York: Wiley; 1980.
-
- Berman AL. The brain stem of the cat: a cytoarchitectonic atlas with stereotaxic coordinates. Madison, WI: University of Wisconsin; 1968.
-
- Bernasconi C, Von Stein A, Chiang C, Konig P. Bidirectional interactions between visual areas in the awake behaving cat. Neuroreport. 2000;11:689–692. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
