TLR3-stimulated dendritic cells up-regulate B7-H1 expression and influence the magnitude of CD8 T cell responses to tumor vaccination
- PMID: 19710456
- PMCID: PMC2789393
- DOI: 10.4049/jimmunol.0900974
TLR3-stimulated dendritic cells up-regulate B7-H1 expression and influence the magnitude of CD8 T cell responses to tumor vaccination
Abstract
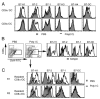
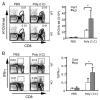
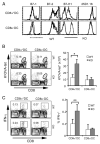
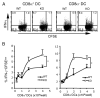
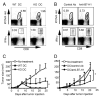
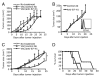
Agonists of TLR have been explored as vaccine adjuvants for tumor immunotherapy. However, their immunological consequences are not fully understood. Although TLR signaling increases the functional potential of dendritic cells (DCs) for priming T cells, coinduction of potentially negative immunoregulatory capacities may impair effector T cell generation. We examined the expression and function of B7 family costimulatory molecules on DCs after activation with the TLR3 agonist, polyinosinic:polycytidylic acid. We demonstrated that polyinosinic:polycytidylic acid consistently up-regulated both B7-2 and B7-H1 molecules on resident, migratory DCs from spleen and lymph nodes. Depletion or blockade of B7-H1 on activated DCs increased the magnitude of effector CD8 T cell expansion. DC-based or protein-based tumor vaccines, in combination with B7-H1 blockade, induced strong effector CD8 T cell responses, resulting in protective immunity against newly established tumors. Our studies suggest that TLR3 signaling has the potential to up-regulate both positive and negative coregulatory molecules on APCs. Selective blockade of negative regulatory molecules in combination with TLR3 agonist may be an effective strategy for increasing the efficacy of tumor vaccines.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

