Deficiency of endothelial heparan sulfates attenuates allergic airway inflammation
- PMID: 19710461
- PMCID: PMC2872128
- DOI: 10.4049/jimmunol.0901604
Deficiency of endothelial heparan sulfates attenuates allergic airway inflammation
Abstract
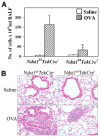
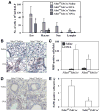
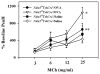
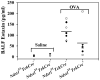
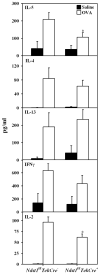
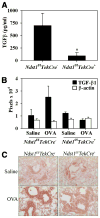
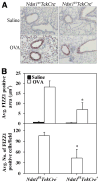
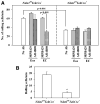
The effect of targeted inactivation of the gene encoding N-deacetylase/N-sulfotransferase-1 (Ndst1), a key enzyme involved in the biosynthesis of heparan sulfate (HS) chains, on the inflammatory response associated with allergic inflammation in a murine model of OVA-induced acute airway inflammation was investigated. OVA-exposed Ndst1(f/f)TekCre(+) (mutant) mice deficient in endothelial and leukocyte Ndst1 demonstrated significantly decreased allergen-induced airway hyperresponsiveness and inflammation characterized by a significant reduction in airway recruitment of inflammatory cells (eosinophils, macrophages, neutrophils, and lymphocytes), diminished IL-5, IL-2, TGF-beta1, and eotaxin levels, as well as decreased expression of TGF-beta1 and the angiogenic protein FIZZ1 (found in inflammatory zone 1) in lung tissue compared with OVA-exposed Ndst1(f/f)TekCre(-) wild-type littermates. Furthermore, murine eosinophils demonstrated significantly decreased rolling on lung endothelial cells (ECs) from mutant mice compared with wild-type ECs under conditions of flow in vitro. Treatment of wild-type ECs, but not eosinophils, with anti-HS Abs significantly inhibited eosinophil rolling, mimicking that observed with Ndst1-deficient ECs. In vivo, trafficking of circulating leukocytes in lung microvessels of allergen-challenged Ndst1-deficient mice was significantly lower than that observed in corresponding WT littermates. Endothelial-expressed HS plays an important role in allergic airway inflammation through the regulation of recruitment of inflammatory cells to the airways by mediating interaction of leukocytes with the vascular endothelium. Furthermore, HS may also participate by sequestering and modulating the activity of allergic asthma-relevant mediators such as IL-5, IL-2, and TGF-beta1.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures
References
-
- Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 2007;446:1030–1037. - PubMed
-
- Kjellén L. Glucosaminyl N-deacetylase/N-sulphotransferases in heparan sulphate biosynthesis and biology. Biochem Soc Trans. 2003;31:340–342. - PubMed
-
- Pallerla SR, Pan Y, Zhang X, Esko JD, Grobe K. Heparan sulfate Ndst1 gene function variably regulates multiple signaling pathways during mouse development. Dev Dyn. 2007;236:556–563. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

