Glyoxalase I gene deletion mutants of Leishmania donovani exhibit reduced methylglyoxal detoxification
- PMID: 19710909
- PMCID: PMC2728510
- DOI: 10.1371/journal.pone.0006805
Glyoxalase I gene deletion mutants of Leishmania donovani exhibit reduced methylglyoxal detoxification
Abstract
Background: Glyoxalase I is a metalloenzyme of the glyoxalase pathway that plays a central role in eliminating the toxic metabolite methyglyoxal. The protozoan parasite Leishmania donovani possesses a unique trypanothione dependent glyoxalase system.
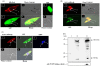
Principal findings: Analysis of the L. donovani GLOI sequence predicted a mitochondrial targeting sequence, suggesting that the enzyme is likely to be targeted to the mitochondria. In order to determine definitively the intracellular localization of GLOI in L. donovani, a full-length GLOI gene was fused to green fluorescent protein (GFP) gene to generate a chimeric construct. Confocal microscopy of L. donovani promastigotes carrying this chimeric construct and immunofluorescence microscopy using anti-GLOI antibodies demonstrated that GLOI is localized in the kinetoplast of the parasite apart from the cytosol. To study the physiological role of GLOI in Leishmania, we first created promastigote mutants heterozygous for GLOI by targeted gene replacement using either hygromycin or neomycin phosphotransferases as selectable markers. Heterozygous mutants of L. donovani display a slower growth rate, have lower glyoxalase I activity and have reduced ability to detoxify methylglyoxal in comparison to the wild-type parasites. Complementation of the heterozygous mutant with an episomal GLOI construct showed the restoration of heterozygous mutant phenotype nearly fully to that of the wild-type. Null mutants were obtained only after GLOI was expressed from an episome in heterozygous mutants.
Conclusions: We for the first time report localization of GLOI in L. donovani in the kinetoplast. To study the physiological role of GLOI in Leishmania, we have generated GLOI attenuated strains by targeted gene replacement and report that GLOI is likely to be an important gene since GLOI mutants in L. donovani showed altered phenotype. The present data supports that the GLOI plays an essential role in the survival of this pathogenic organism and that inhibition of the enzyme potentiates the toxicity of methylglyoxal.
Conflict of interest statement
Figures



Similar articles
-
Methylglyoxal metabolism in trypanosomes and leishmania.Semin Cell Dev Biol. 2011 May;22(3):271-7. doi: 10.1016/j.semcdb.2011.02.001. Epub 2011 Feb 15. Semin Cell Dev Biol. 2011. PMID: 21310261 Free PMC article. Review.
-
Glyoxalase I from Leishmania donovani: a potential target for anti-parasite drug.Biochem Biophys Res Commun. 2005 Dec 2;337(4):1237-48. doi: 10.1016/j.bbrc.2005.09.179. Epub 2005 Oct 7. Biochem Biophys Res Commun. 2005. PMID: 16236261
-
A glutathione-specific aldose reductase of Leishmania donovani and its potential implications for methylglyoxal detoxification pathway.Gene. 2009 Jan 15;429(1-2):1-9. doi: 10.1016/j.gene.2008.09.037. Epub 2008 Oct 15. Gene. 2009. PMID: 18983902
-
Characterization of the gene encoding glyoxalase II from Leishmania donovani: a potential target for anti-parasite drugs.Biochem J. 2006 Jan 1;393(Pt 1):227-34. doi: 10.1042/BJ20050948. Biochem J. 2006. PMID: 16159313 Free PMC article.
-
Glyoxalase pathway of trypanosomatid parasites: a promising chemotherapeutic target.Curr Drug Targets. 2008 Nov;9(11):957-65. doi: 10.2174/138945008786786082. Curr Drug Targets. 2008. PMID: 18991608 Review.
Cited by
-
The role of glyoxalases for sugar stress and aging, with relevance for dyskinesia, anxiety, dementia and Parkinson's disease.Aging (Albany NY). 2011 Jan;3(1):5-9. doi: 10.18632/aging.100258. Aging (Albany NY). 2011. PMID: 21248374 Free PMC article. No abstract available.
-
Identification and functional characterization of a novel bacterial type asparagine synthetase A: a tRNA synthetase paralog from Leishmania donovani.J Biol Chem. 2014 Apr 25;289(17):12096-12108. doi: 10.1074/jbc.M114.554642. Epub 2014 Mar 7. J Biol Chem. 2014. PMID: 24610810 Free PMC article.
-
Genetically modified organisms and visceral leishmaniasis.Front Immunol. 2014 May 14;5:213. doi: 10.3389/fimmu.2014.00213. eCollection 2014. Front Immunol. 2014. PMID: 24860575 Free PMC article. Review.
-
Characteristic Variations and Similarities in Biochemical, Molecular, and Functional Properties of Glyoxalases across Prokaryotes and Eukaryotes.Int J Mol Sci. 2017 Mar 30;18(4):250. doi: 10.3390/ijms18040250. Int J Mol Sci. 2017. PMID: 28358304 Free PMC article. Review.
-
Methylglyoxal metabolism in trypanosomes and leishmania.Semin Cell Dev Biol. 2011 May;22(3):271-7. doi: 10.1016/j.semcdb.2011.02.001. Epub 2011 Feb 15. Semin Cell Dev Biol. 2011. PMID: 21310261 Free PMC article. Review.
References
-
- Carrington SJ, Douglas KT. The glyoxalase enigma—the biological consequences of a ubiquitous enzyme. IRCS Med Sci. 1986;14:763–768.
-
- Thornalley PJ. Pharmacology of methylglyoxal: formation, modification of proteins and nucleic acids and enzymatic detoxification a role in pathogenesis and antiproliferative chemotherapy. Gen Pharmacol. 1996;27:565–573. - PubMed
-
- Irsch T, Krauth-Siegel RL. Glyoxalase II of African Trypanosomes is trypanothione-dependent. J Biol Chem. 2004;279:22209–22217. - PubMed
-
- Padmanabhan KP, Mukherjee A, Singh S, Chattopadhyaya S, Gowri VS, et al. Glyoxalase I from Leishmania donovani: A potential target for anti-parasite drug. Biochem Biophys Res Commun. 2005;337:1237–1248. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

