Efficacy of targeted therapy in patients with renal cell carcinoma with pre-existing or new bone metastases
- PMID: 19711099
- PMCID: PMC11828039
- DOI: 10.1007/s00432-009-0664-7
Efficacy of targeted therapy in patients with renal cell carcinoma with pre-existing or new bone metastases
Abstract
Introduction: This single-centre retrospective analysis of data from three randomised studies and two expanded-access studies compared the effect of interferon (IFN)-alfa, sunitinib, and sorafenib on the occurrence and progression of metastatic bone lesions in patients with renal cell carcinoma (RCC).
Methods: The analysis included 292 patients: 107 received sunitinib 50 mg/day in 6-week cycles (Schedule 4/2), 147 received sorafenib 800 mg/day, and 38 received placebo or IFN-alfa 9 MU t.i.w.
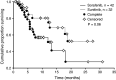
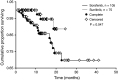
Results: Pre-existing metastatic bone lesions were reported in 82 patients, of which 30 experienced progression. Twenty-three of 210 patients developed new bone lesions. Overall, sunitinib appeared slightly more effective than sorafenib or IFN-alfa at extending mean time to progression of pre-existing bone lesions (P = 0.057). Compared with sorafenib, sunitinib significantly decreased formation (P = 0.034) and prolonged time to occurrence of new bone lesions (P = 0.047).
Conclusion: Further evaluation of the effect of these therapies on bone metastases in RCC is warranted.
Figures
References
-
- Abrams TJ, Lee LB, Murray LJ, Pryer NK, Cherrington JM (2003) SU11248 inhibits KIT and platelet-derived growth factor receptor beta in preclinical models of human small cell lung cancer. Mol Cancer Ther 2:471–478 - PubMed
-
- Beeram M, Patnaik A, Rowinsky EK (2005) Raf: a strategic target for therapeutic development against cancer. J Clin Oncol 23:6771–6790 - PubMed
-
- Bukowski R, Szczylik C, Stadler W, Simantov R, Shan M (2007) Final results of the randomized phase III trial of sorafenib in advanced renal cell carcinoma: survival and biomarker analysis. J Clin Oncol 25(18S):240s (Abstract 5023)
-
- Coleman RE (1997) Skeletal complications of malignancy. Cancer 80:1588–1594 - PubMed
-
- Costa L, Major PP (2009) Effect of bisphosphonates on pain and quality of life in patients with bone metastases. Nat Clin Pract Oncol 6:163–174 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials