Stromal cell-derived CSF-1 blockade prolongs xenograft survival of CSF-1-negative neuroblastoma
- PMID: 19711348
- PMCID: PMC3222589
- DOI: 10.1002/ijc.24859
Stromal cell-derived CSF-1 blockade prolongs xenograft survival of CSF-1-negative neuroblastoma
Abstract
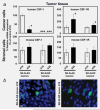
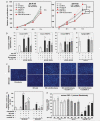
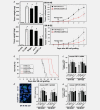
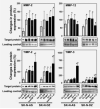
The molecular mechanisms of tumor-host interactions that render neuroblastoma (NB) cells highly invasive are unclear. Cancer cells upregulate host stromal cell colony-stimulating factor-1 (CSF-1) production to recruit tumor-associated macrophages (TAMs) and accelerate tumor growth by affecting extracellular matrix remodeling and angiogenesis. By coculturing NB with stromal cells in vitro, we showed the importance of host CSF-1 expression for macrophage recruitment to NB cells. To examine this interaction in NB in vivo, mice bearing human CSF-1-expressing SK-N-AS and CSF-1-negative SK-N-DZ NB xenografts were treated with intratumoral injections of small interfering RNAs directed against mouse CSF-1. Significant suppression of both SK-N-AS and SK-N-DZ NB growth by these treatments was associated with decreased TAM infiltration, matrix metalloprotease (MMP)-12 levels and angiogenesis compared to controls, while expression of tissue inhibitors of MMPs increased following mouse CSF-1 blockade. Furthermore, Tie-2-positive and -negative TAMs recruited by host CSF-1 were identified in NB tumor tissue by confocal microscopy and flow cytometry. However, host-CSF-1 blockade prolonged survival only in CSF-1-negative SK-N-DZ NB. These studies demonstrated that increased CSF-1 production by host cells enhances TAM recruitment and NB growth and that the CSF-1 phenotype of NB tumor cells adversely affects survival.
Figures



References
-
- Brodeur G, Maris J. Neuroblastoma. In: Pizzo P, Poplack DG, editors. Cytokine reference. 4th edn. Academic press; London: 2002. pp. 895–938.
-
- van Noesel MM, Versteeg R. Pediatric neuroblastomas: genetic and epigenetic ‘danse macabre.’. Gene. 2004;325:1–15. - PubMed
-
- Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–16. - PubMed
-
- Goldsmith KC, Hogarty MD. Targeting programmed cell death pathways with experimental therapeutics: opportunities in high-risk neuroblastoma. Cancer Lett. 2005;228:133–41. - PubMed
-
- Nilsson JA, Keller UB, Baudino TA, Yang C, Norton S, Old JA, Nilsson LM, Neale G, Kramer DL, Porter CW, Cleveland JL. Targeting ornithine decarboxylase in Myc-induced lymphomagenesis prevents tumor formation. Cancer Cell. 2005;7:433–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

