Regio- and stereoselective synthesis of fluoroalkenes by directed Au(I) catalysis
- PMID: 19711904
- PMCID: PMC2753819
- DOI: 10.1021/ol9016782
Regio- and stereoselective synthesis of fluoroalkenes by directed Au(I) catalysis
Abstract
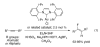
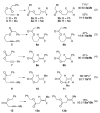
Au-catalyzed hydrofluorination reactions of a range of functionalized alkynes are reported. In the presence of an appropriate directing group, localized with particular spacing from the pendant alkyne, regioselective and predictable conversion of the alkyne to the Z-vinyl fluoride may be achieved. In selected cases, yields and selectivities are excellent. Additional experiments with two directing groups installed have established some initial principles with respect to a hierarchy of directing groups and their capacity for influencing hydrofluorination regioselectivity.
Figures



References
-
- Cox JD, Gundry HA, Head AJ. T. Faraday Soc. 1964;60:653.
-
- Bondi A. J. Phys. Chem. 1964;68:441.
-
- Banks HD. J. Org. Chem. 2006;71:8089. - PubMed
-
- Böhm H,-J, Banner D, Bendels S, Kansy M, Kuhn B, Müller K, Obst-Sander U, Stahl M. ChemBioChem. 2004;5:637. - PubMed
-
- Garrett GS, Emge TJ, Lee SC, Fischer EM, Dyehouse K, McIver JM. J. Org. Chem. 1991;56:4823.
- Bartlett PA, Otake A. J. Org. Chem. 1995;60:3107.
- Otaka A, Mitsuyama E, Watanabe J, Watanabe H, Fujii N. Biopolymers. 2004;76:140. - PubMed
- Urban JJ, Tillman BG, Cronin WA. J. Phys. Chem. A. 2006;110:11120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

