Differential regulation of human PlGF gene expression in trophoblast and nontrophoblast cells by oxygen tension
- PMID: 19712973
- PMCID: PMC2768061
- DOI: 10.1016/j.placenta.2009.08.003
Differential regulation of human PlGF gene expression in trophoblast and nontrophoblast cells by oxygen tension
Abstract
Objective: To determine the mechanism for differential effects of low oxygen tension on human PlGF gene transcription in trophoblast and nontrophoblast cells.
Study design: Human PlGF reporter clones and real-time RT-PCR were used to compare the effects of hypoxia on gene transcription in human trophoblast and nontrophoblast cell lines. Overexpression of HIF-1alpha, inhibition of HIF-1 function and biochemical assessments of HIF-1 co-factor interactions were used to characterize hypoxia response mechanisms regulating PlGF transcription.
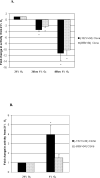
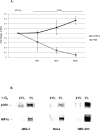
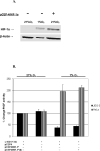
Results: PlGF transcription is specifically inhibited by low oxygen tension in trophoblast but is induced in some nontrophoblast cells. Overexpression of HIF-1alpha in normoxic cells or inhibition of HIF-1 function in hypoxic cells did not significantly alter transcription patterns of the PlGF gene in either cell type.
Conclusions: These results suggest that transcriptional repression of PlGF gene expression occurs in human trophoblast exposed to low oxygen tension but that PlGF transcription is stimulated in certain hypoxic nontrophoblast cells. However, regulation of PlGF transcription is not mediated by functional HIF-1 activity in either cell type.
Figures


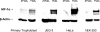


References
-
- Ziche M, Maglione D, Ribatti D, Morbidelli L, Lago CT, Battisti M, Paoletti I, Barra A, Tucci M, Parise G, Vincenti V, Granger HJ, Viglietto G, Persico MG. Placenta growth factor-1 is chemotactic, mitogenic, and angiogenic. Lab Invest. 1997;76:517–31. - PubMed
-
- Khaliq A, Li XF, Shams M, Sisi P, Acevedo CA, Whittle MJ, Weich H, Ahmed A. Localisation of placenta growth factor (PIGF) in human term placenta. Growth Factors. 1996;13:243–50. - PubMed
-
- Clark DE, Smith SK, Licence D, Evans AL, Charnock-Jones DS. Comparison of expression patterns for placenta growth factor, vascular endothelial growth factor (VEGF), VEGF-B and VEGF-C in the human placenta throughout gestation. J Endocrinol. 1998;159:459–67. - PubMed
-
- Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, Gazit R, Yutkin V, Benharroch D, Porgador A, Keshet E, Yagel S, Mandelboim O. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12:1065–1074. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

