Functional characterization of human cytochrome P450 2S1 using a synthetic gene-expressed protein in Escherichia coli
- PMID: 19713358
- PMCID: PMC2774995
- DOI: 10.1124/mol.109.057752
Functional characterization of human cytochrome P450 2S1 using a synthetic gene-expressed protein in Escherichia coli
Abstract
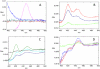
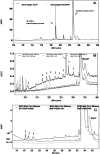
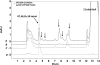
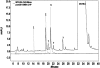
Human cytochrome P450 2S1 was recently identified and shown to be inducible by 2,3,7,8-tetrachlorodibenzo-p-dioxin and hypoxia. It is highly expressed in epithelial cells of tissues that are exposed to the environment and in many tumors of epithelial origin. The biological function of CYP2S1 has not yet been determined, although its possible role in carcinogen metabolism has been suggested. In this report, we investigated its ability to metabolize carcinogens. To obtain a large quantity of active enzyme for substrate screening, we overexpressed CYP2S1 in Escherichia coli (200 nM culture), using a synthetic gene approach. High-level expression allowed us to achieve purification of CYP2S1 with high specific content and purity (16 nmol/mg). Despite high-level expression, we found that CYP2S1 was not readily reduced by cytochrome P450 reductase, and thus no activity was found using NADPH. However, the oxidative activity of CYP2S1 was supported by cumene hydroperoxide or H(2)O(2), such that CYP2S1 oxidized many important environmental carcinogens, including benzo[a]pyrene, 9,10-dihydro-benzo[a]pyrene, 7,12-dimethylbenz[a]anthracene, benzo[a]pyrene-7,8-dihydrodiol, aflatoxin B1, naphthalene, and styrene, with high turnover. Most substrates tested were converted to detoxification products, except in the case of benzo[a]pyrene-7,8-dihydrodiol, which was converted into the very potent carcinogenic metabolite 7,8-dihydrodiol-trans-9,10-epoxide at a relatively efficient rate (K(m) = 12.4 +/- 2 microM, turnover = 2.3 min(-1)). This metabolite formation was also supported both in vitro and in vivo by fatty acid hydroperoxides described in the accompanying report (p. 1044). Together, these data indicate that CYP2S1 contributes to the metabolism of environmental carcinogens via an NADPH independent activity.
Figures





References
-
- Anari MR, Khan S, Jatoe SD, O'Brien PJ. (1997) Cytochrome P450 dependent xenobiotic activation by physiological hydroperoxides in intact hepatocytes. Eur J Drug Metab Pharmacokinet 22:305–310 - PubMed
-
- Baldwin RM, Shultz MA, Buckpitt AR. (2005) Bioactivation of the pulmonary toxicants naphthalene and 1-nitronaphthalene by rat CYP2F4. J Pharmacol Exp Ther 312:857–865 - PubMed
-
- Barr DP, Martin MV, Guengerich FP, Mason RP. (1996) Reaction of cytochrome P450 with cumene hydroperoxide: ESR spin-trapping evidence for the homolytic scission of the peroxide O-O bond by ferric cytochrome P450 1A2. Chem Res Toxicol 9:318–325 - PubMed
-
- Barr DP, Mason RP. (1995) Mechanism of radical production from the reaction of cytochrome c with organic hydroperoxides. An ESR spin trapping investigation. J Biol Chem 270:12709–12716 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

