B-cell follicle development remodels the conduit system and allows soluble antigen delivery to follicular dendritic cells
- PMID: 19713459
- PMCID: PMC2788973
- DOI: 10.1182/blood-2009-06-229567
B-cell follicle development remodels the conduit system and allows soluble antigen delivery to follicular dendritic cells
Abstract
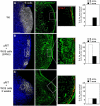
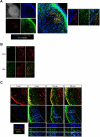
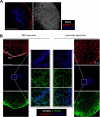
Afferent lymph is transported throughout lymph nodes (LNs) by the conduit system. Whereas this conduit network is dense in the T-cell zone, it is sparse in B-cell follicles. In this study, we show that this differential organization emerges during lymph node development. Neonatal LNs lack B follicles, but have a developed T-cell zone and a dense conduit network. As new T and B cells enter the developing LN, the conduit network density is maintained in the T, but not the B zone, leading to a profound remodeling of the follicular network that nevertheless maintains its connectivity. In adults, the residual follicular conduits transport soluble antigen to deep regions, where follicular dendritic cells are abundant and appear to replace the fibroblastic reticular cells that enwrap conduits in the T zone. This strategic location correlates with the capacity of the follicular dendritic cells to capture antigen even in the absence of antigen-specific antibodies. Together, these results describe how the stromal organization of the T and B regions of LNs diverges during development, giving rise to distinct antigen transport and delivery modes in the 2 compartments.
Figures


References
-
- Willard-Mack CL. Normal structure, function, and histology of lymph nodes. Toxicol Pathol. 2006;34:409–424. - PubMed
-
- Farr AG, Cho Y, De Bruyn PP. The structure of the sinus wall of the lymph node relative to its endocytic properties and transmural cell passage. Am J Anat. 1980;157:265–284. - PubMed
-
- Racz P, Tenner-Racz K, Myrvik QN, Ockers JR. The “mosaic” structure of the sinuses in the hilar lymph node complex of the lungs in rabbits undergoing a pulmonary cell-mediated reaction: an electron microscopic study. J Reticuloendothel Soc. 1978;24:527–545. - PubMed
-
- Clark SL., Jr The reticulum of lymph nodes in mice studied with the electron microscope. Am J Anat. 1962;110:217–257. - PubMed
-
- van Ewijk W, Brekelmans PJ, Jacobs R, Wisse E. Lymphoid microenvironments in the thymus and lymph node. Scanning Microsc. 1988;2:2129–2140. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

