Nonimmune mechanisms of muscle damage in myositis: role of the endoplasmic reticulum stress response and autophagy in the disease pathogenesis
- PMID: 19713850
- PMCID: PMC2877394
- DOI: 10.1097/BOR.0b013e3283319265
Nonimmune mechanisms of muscle damage in myositis: role of the endoplasmic reticulum stress response and autophagy in the disease pathogenesis
Abstract
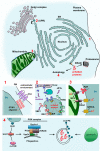
Purpose of review: Recent literature in inflammatory myopathies suggests that both immune (cell-mediated and humoral) and nonimmune [endoplasmic reticulum (ER) stress and autophagy] mechanisms play a role in muscle fiber damage and dysfunction. This review describes these findings and discusses their relevance to disease pathogenesis and therapy.
Recent findings: Recent studies highlight the role of ER stress response, especially the roles of hexose-6-phosphate dehydrogenase and ER-anchored RING finger E3 ligase in the activation of unfolded protein response and the formation of vacuoles and inclusions in myopathies. Several studies investigated the link between inflammation and the beta-amyloid-associated muscle fiber degeneration and loss of muscle function. Likewise, the roles of ER stress and autophagy in skeletal muscle damage have been explored in multiple muscle diseases.
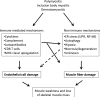
Summary: Current data indicate that the ER stress, nuclear factor-kappaB pathway and autophagy are active in the skeletal muscle of myositis patients, and the proinflammatory nuclear factor-kappaB pathway connects the immune and nonimmune pathways of muscle damage. The relative contributions of each of these pathways to muscle fiber damage are currently unclear. Therefore, further defining the role of these pathways in disease pathogenesis should help to design effective therapeutic agents for these diseases.
Figures
References
-
- DeVere R, Bradley WG. Polymyositis: its presentation, morbidity and mortality. Brain. 1975;98:637–666. - PubMed
-
- Plotz PH, Dalakas M, Leff RL, Love LA, Miller FW, Cronin ME. Current concepts in the idiopathic inflammatory myopathies: polymyositis, dermatomyositis, and related disorders. Ann Intern Med. 1989;111:143–157. - PubMed
-
- Emslie-Smith AM, Arahata K, Engel AG. Major histocompatibility complex class I antigen expression, immunolocalization of interferon subtypes, and T cell-mediated cytotoxicity in myopathies. Hum Pathol. 1989;20:224–231. - PubMed
-
- Englund P, Nennesmo I, Klareskog L, Lundberg IE. Interleukin-1alpha expression in capillaries and major histocompatibility complex class I expression in type II muscle fibers from polymyositis and dermatomyositis patients: important pathogenic features independent of inflammatory cell clusters in muscle tissue. Arthritis Rheum. 2002;46:1044–1055. - PubMed
-
- Adams E. A pilot study : use of fludarabine for refractory dermatomyositis and polymyositis, and examination of endpoint measures. J Rheumatol. 1999;26(2):352–360. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

