Modulation of intracellular ROS levels by TIGAR controls autophagy
- PMID: 19713938
- PMCID: PMC2736014
- DOI: 10.1038/emboj.2009.242
Modulation of intracellular ROS levels by TIGAR controls autophagy
Abstract
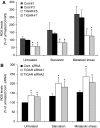
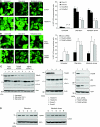
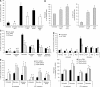
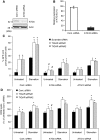
The p53-inducible TIGAR protein functions as a fructose-2,6-bisphosphatase, promoting the pentose phosphate pathway and helping to lower intracellular reactive oxygen species (ROS). ROS functions in the regulation of many cellular responses, including autophagy--a response to stress conditions such as nutrient starvation and metabolic stress. In this study, we show that TIGAR can modulate ROS in response to nutrient starvation or metabolic stress, and functions to inhibit autophagy. The ability of TIGAR to limit autophagy correlates strongly with the suppression of ROS, with no clear effects on the mTOR pathway, and is p53 independent. The induction of autophagy in response to loss of TIGAR can function to moderate apoptotic response by restraining ROS levels. These results reveal a complex interplay in the regulation of ROS, autophagy and apoptosis in response to TIGAR expression, and shows that proteins similar to TIGAR that regulate glycolysis can have a profound effect on the autophagic response through ROS regulation.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Averous J, Proud CG (2006) When translation meets transformation: the mTOR story. Oncogene 25: 6423–6435 - PubMed
-
- Azad MB, Chen Y, Gibson SB (2009) Regulation of autophagy by reactive oxygen species (ROS): implications for cancer progression and treatment. Antioxid Redox Signal 11: 777–790 - PubMed
-
- Balaban RS, Nemoto S, Finkel T (2005) Mitochondria, oxidants, and aging. Cell 120: 483–495 - PubMed
-
- Bampton ET, Goemans CG, Niranjan D, Mizushima N, Tolkovsky AM (2005) The dynamics of autophagy visualized in live cells: from autophagosome formation to fusion with endo/lysosomes. Autophagy 1: 23–36 - PubMed
-
- Bensaad K, Tsuruta A, Selak MA, Vidal MN, Nakano K, Bartrons R, Gottlieb E, Vousden KH (2006) TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell 126: 107–120 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

