Regulation of host translational machinery by African swine fever virus
- PMID: 19714237
- PMCID: PMC2727446
- DOI: 10.1371/journal.ppat.1000562
Regulation of host translational machinery by African swine fever virus
Abstract
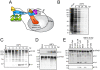
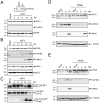
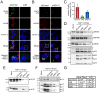
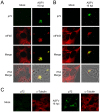
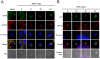
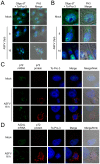
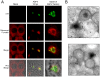
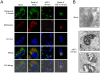
African swine fever virus (ASFV), like other complex DNA viruses, deploys a variety of strategies to evade the host's defence systems, such as inflammatory and immune responses and cell death. Here, we analyse the modifications in the translational machinery induced by ASFV. During ASFV infection, eIF4G and eIF4E are phosphorylated (Ser1108 and Ser209, respectively), whereas 4E-BP1 is hyperphosphorylated at early times post infection and hypophosphorylated after 18 h. Indeed, a potent increase in eIF4F assembly is observed in ASFV-infected cells, which is prevented by rapamycin treatment. Phosphorylation of eIF4E, eIF4GI and 4E-BP1 is important to enhance viral protein production, but is not essential for ASFV infection as observed in rapamycin- or CGP57380-treated cells. Nevertheless, eIF4F components are indispensable for ASFV protein synthesis and virus spread, since eIF4E or eIF4G depletion in COS-7 or Vero cells strongly prevents accumulation of viral proteins and decreases virus titre. In addition, eIF4F is not only activated but also redistributed within the viral factories at early times of infection, while eIF4G and eIF4E are surrounding these areas at late times. In fact, other components of translational machinery such as eIF2alpha, eIF3b, eIF4E, eEF2 and ribosomal P protein are enriched in areas surrounding ASFV factories. Notably, the mitochondrial network is polarized in ASFV-infected cells co-localizing with ribosomes. Thus, translation and ATP synthesis seem to be coupled and compartmentalized at the periphery of viral factories. At later times after ASFV infection, polyadenylated mRNAs disappear from the cytoplasm of Vero cells, except within the viral factories. The distribution of these pools of mRNAs is similar to the localization of viral late mRNAs. Therefore, degradation of cellular polyadenylated mRNAs and recruitment of the translation machinery to viral factories may contribute to the inhibition of host protein synthesis, facilitating ASFV protein production in infected cells.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Mohr I. Phosphorylation and dephosphorylation events that regulate viral mRNA translation. Virus Res. 2006;119:89–99. - PubMed
-
- Schneider RJ, Mohr I. Translation initiation and viral tricks. Trends Biochem Sci. 2003;28:130–136. - PubMed
-
- Prevot D, Darlix JL, Ohlmann T. Conducting the initiation of protein synthesis: the role of eIF4G. Biol Cell. 2003;95:141–156. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

