Solid-Phase Synthesis of Heterobivalent Ligands Targeted to Melanocortin and Cholecystokinin Receptors
- PMID: 19714261
- PMCID: PMC2732021
- DOI: 10.1007/s10989-008-9150-3
Solid-Phase Synthesis of Heterobivalent Ligands Targeted to Melanocortin and Cholecystokinin Receptors
Abstract
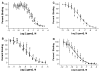
Heteromultivalency provides a route to increase binding avidity and to high specificity when compared to monovalent ligands. The enhanced specificity can potentially serve as a unique platform to develop diagnostics and therapeutics. To develop new imaging agents based upon multivalency, we employed heterobivalent constructs of optimized ligands. In this report, we describe synthetic methods we have developed for the preparation of heterobivalent constructs consisting of ligands targeted simultaneously to the melanocortin receptor, hMC4R, and the cholecystokinin receptors, CCK-2R. Modeling data suggest that a linker distance span of 20-50 Å is needed to crosslink these two G-protein coupled receptors (GPCRs). The two ligands were tethered with linkers of varying rigidity and length, and flexible polyethylene glycol based PEGO chain or semi-rigid [poly(Pro-Gly)] linkers were employed for this purpose. The described synthetic strategy provides a modular way to assemble ligands and linkers on solid-phase supports. Examples of heterobivalent ligands are provided to illustrate the increased binding avidity to cells that express the complementary receptors.
Figures



References
-
- Caplan MR, Rosca EV. Targeting drugs to combinations of receptors: a modeling analysis of potential specificity. Ann Biomed Eng. 2005;33:1113–1124. - PubMed
-
- Fourmy D, Escrieut C, Archer E, et al. Structure of cholecystokinin receptor binding sites and mechanism of activation/inactivation by agonists/antagonists. Pharmacol Toxicol. 2002;91:313–320. - PubMed
-
- Gillies RJ, Hruby VJ. Expression-driven reverse engineering of targeted imaging and therapeutic agents. Expert Opin Ther Targets. 2003;7:137–139. - PubMed
-
- Handl H, Gillies RJ. Lanthanide-based luminescent assays for ligand-receptor interactions. Life Sci. 2005;77:361–371. - PubMed
-
- Handl HL, Vagner J, Han HY, Mash E, Hruby VJ, Gillies RJ. Hitting multiple targets with multimeric ligands. Expert Opin Ther Targets. 2004a;8:565–586. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
