A phase II, randomized, double-blind, placebo-controlled, dose-ranging study of belimumab in patients with active systemic lupus erythematosus
- PMID: 19714604
- PMCID: PMC2758229
- DOI: 10.1002/art.24699
A phase II, randomized, double-blind, placebo-controlled, dose-ranging study of belimumab in patients with active systemic lupus erythematosus
Abstract
Objective: To assess the safety, tolerability, biologic activity, and efficacy of belimumab in combination with standard of care therapy (SOC) in patients with active systemic lupus erythematosus (SLE).
Methods: Patients with a Safety of Estrogens in Lupus Erythematosus: National Assessment (SELENA) version of the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) score >/=4 (n = 449) were randomly assigned to belimumab (1, 4, or 10 mg/kg) or placebo in a 52-week study. Coprimary end points were the percent change in the SELENA-SLEDAI score at week 24 and the time to first SLE flare.
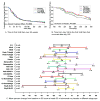
Results: Significant differences between the treatment and placebo groups were not attained for either primary end point, and no dose response was observed. Reductions in SELENA-SLEDAI scores from baseline were 19.5% in the combined belimumab group versus 17.2% in the placebo group. The median time to first SLE flare was 67 days in the combined belimumab group versus 83 days in the placebo group. However, the median time to first SLE flare during weeks 24-52 was significantly longer with belimumab treatment (154 versus 108 days; P = 0.0361). In the subgroup (71.5%) of serologically active patients (antinuclear antibody titer >/=1:80 and/or anti-double-stranded DNA [anti-dsDNA] >/=30 IU/ml), belimumab treatment resulted in significantly better responses at week 52 than placebo for SELENA-SLEDAI score (-28.8% versus -14.2%; P = 0.0435), physician's global assessment (-32.7% versus -10.7%; P = 0.0011), and Short Form 36 physical component score (+3.0 versus +1.2 points; P = 0.0410). Treatment with belimumab resulted in a 63-71% reduction of naive, activated, and plasmacytoid CD20+ B cells, and a 29.4% reduction in anti-dsDNA titers (P = 0.0017) by week 52. The rates of adverse events and serious adverse events were similar in the belimumab and placebo groups.
Conclusion: Belimumab was biologically active and well tolerated. The effect of belimumab on the reduction of SLE disease activity or flares was not significant. However, serologically active SLE patients responded significantly better to belimumab therapy plus SOC than to SOC alone.
Trial registration: ClinicalTrials.gov NCT00071487.
Figures

Comment in
-
Global versus organ-specific outcome measures in systemic lupus erythematosus: comment on the articles by Furie et al, Nikpour et al, Wallace et al, Burgos et al, and Ramos-Casals et al.Arthritis Care Res (Hoboken). 2010 Apr;62(4):580-1; author replies 581-2. doi: 10.1002/acr.20053. Arthritis Care Res (Hoboken). 2010. PMID: 20391517 No abstract available.
References
-
- Moore PA, Belvedere O, Orr A, Pieri K, LaFleur DW, Feng P, et al. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285(5425):260–3. - PubMed
-
- Gross JA, Johnston J, Mudri S, Enselman R, Dillon SR, Madden K, et al. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. 2000;404(6781):995–9. - PubMed
-
- Yan M, Brady JR, Chan B, Lee WP, Hsu B, Harless S, et al. Identification of a novel receptor for B lymphocyte stimulator that is mutated in a mouse strain with severe B cell deficiency. Curr Biol. 2001;11(19):1547–52. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

